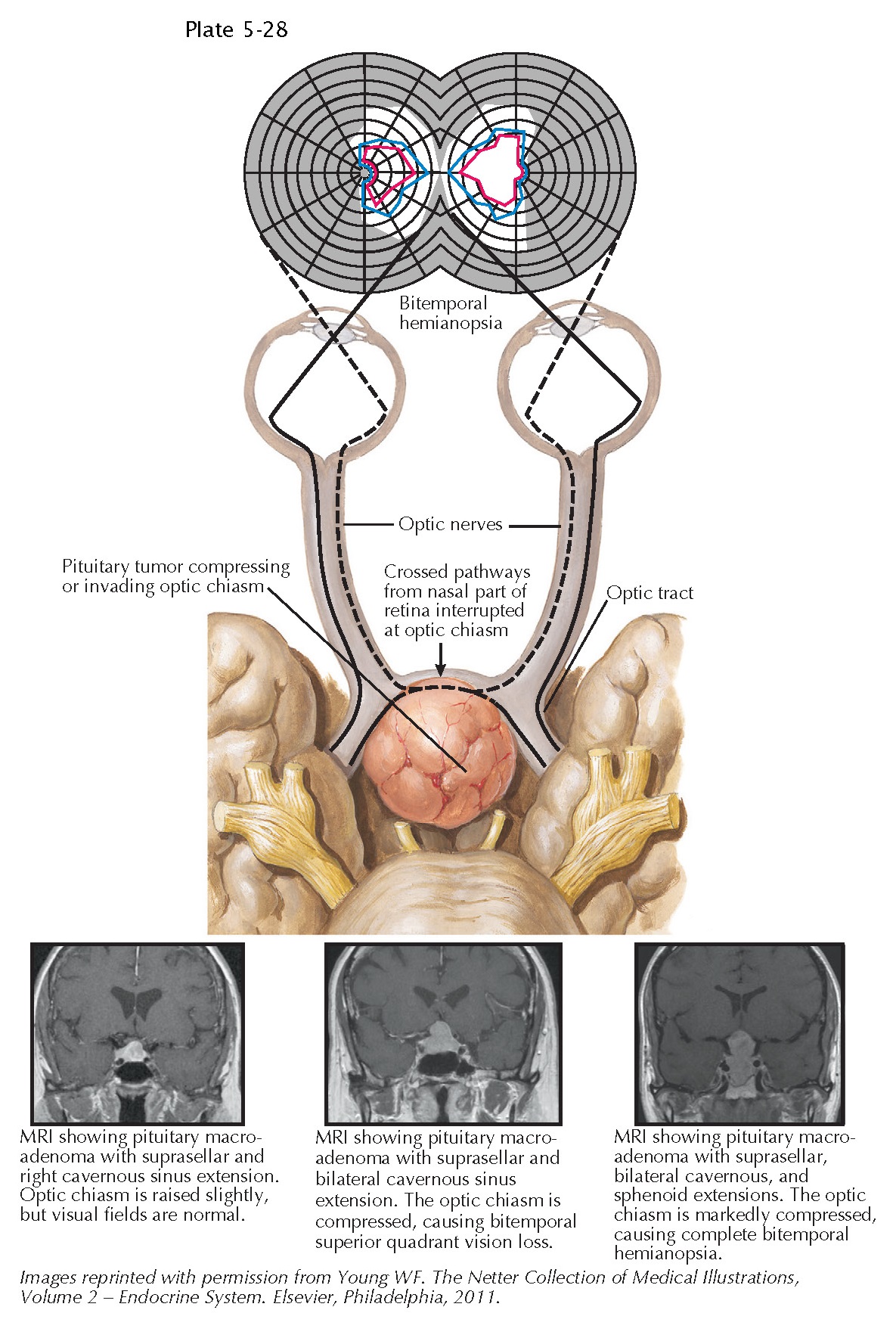
Effects of Pituitary Mass Lesions on the Visual Apparatus
The close anatomic relationship between the pituitary and the optic apparatus, notably the optic chiasm, but also the prechiasmatic optic nerves and the postchiasmatic optic tracts, accounts for the frequent occurrence of visual deficits in patients with large pituitary mass lesions extending superiorly. At the optic chiasm, axons of the retinal ganglion cells that originate in the nasal portion of each retina cross to the contralateral side. In contrast, nerve fibers from the temporal portion of each retina remain on the ipsilateral side past the chiasm to form each optic tract, accompanied by nerve fibers crossing from the nasal portion of the contralateral retina.
A variety of
mass lesions may arise within the sella. In addition to benign pituitary
adenomas, which account for approximately 90% of mass lesions in surgical
series, there are a large number of pathologic sellar mass lesions. These
include benign (craniopha-ryngioma, meningioma) and malignant (chondrosarcoma,
lymphoma, metastases, or the exceedingly rare primary pituitary
carcinoma) neoplasms, cystic lesions (Rathke’s cleft cyst;
arachnoid, dermoid, and epidermoid cyst), vascular pathologies
(aneurysms, arteriovenous malformations), inflammatory lesions (primary
or secondary hypophysitis), infection, or pituitary hyperplasia.
Mass lesions
extending
superiorly from the sella often impinge on the optic chiasm, which is generally
located directly above the diaphragma sellae (in approximately 90% of
individuals). Early abnormalities that occur as a result of chiasmatic
compression include loss of color perception as a result of optic neuropathy,
which can be documented using standard Ishihara chart testing, as well as
variable loss of peripheral (temporal) field vision. Among patients with mass lesions
growing from the sella, vision is generally lost first in either or both superior
temporal quadrants. In contrast, mass lesions arising at the base of the
hypothalamus, which compress the optic chiasm from above, may lead
to early loss of vision in the inferior temporal quadrants.
Compression of
the prechiasmatic optic nerves may lead to ipsilateral optic
neuropathy, giving rise to a central scotoma. Lesions that compress the anterior
portion of the chiasm on one side may give rise to ipsilateral optic
neuropathy (central scotoma) and loss of peripheral vision in the
contralateral superior temporal quadrant, a constellation termed “junctional
scotoma.” More posteriorly located lesions may impinge upon one of
the optic tracts, leading to contralateral homonymous hemianopsia.
Preliminary visual field testing may be conducted using bedside confrontation testing, but definitive evaluation of peripheral vision requires formal perimetry, using either an automated (Humphrey) or manual (Goldmann) method. Primary optic atrophy is present in cases of long-standing nerve fiber compression. Papilledema may rarely occur in patients with very large tumors extending toward the third ventricle, causing obstructive hydrocephalus. Consultation with an experi- enced neuro-ophthalmologist is advised for patients with mass lesions abutting or compressing the optic apparatus. Recovery of visual function occurs with relief of compression of the optic apparatus in most patients (approximately 70% to 75% of cases). However, the likelihood and extent of visual recovery are generally higher with shorter duration of nerve fiber compression. Thus early diagnosis and prompt decompression (generally via surgery, but also medical therapy in patients with prolactin-secreting adenomas) are very important to optimize visual outcomes in these patients.