Cardiac
Catheterization
VASCULAR ACCESS AND RIGHT-SIDED HEART CATHETERIZATION
Cardiac catheterization, first attempted by Forssmann on himself in 1928, was developed by Cournand, Richards, and their colleagues and is now a common procedure in both clinical and research laboratories.
The primary goal
of right-sided heart catheterization is to access the conditions existing in the
chambers and great vessels of the right side of the heart. In these procedures a
radiopaque flexible catheter of various designs, including balloon tipped, is introduced
into a vein, usually the femoral or jugular, percutaneously using local anesthesia.
After introduction into the vein, the catheter is manipulated under fluoroscopic
control and constant electrocardiographic monitoring down-stream through the venous
system to the right atrium, and eventually into the right ventricle and pulmonary
artery. The catheter is often wedged and is advanced into the most peripheral
branch of the pulmonary artery that will accept the catheter tip or occlusion by
a balloon-tipped catheter.. A pressure recorded from the wedge position has essentially
the same mean pressure as the left atrium and the same but delayed phasic features.
If there is no mitral stenosis, the pulmonary capillary wedge pressure (PCWP) reflects
the left ventricular end-diastolic pressure (LVEDP).
The position of
the catheter in the fluoroscopic image may indicate some departure from the intracardiac
course normally taken by a catheter. Examples include passage into a persistent
left superior vena cava through the coronary sinus from the right atrium, passage
through a patent ductus arteriosus, and traversal of an interatrial or interventricular
septal defect.
Blood can be sampled
for oxygen or other analysis, and pressures can be measured through the catheter
from any point reached. Oxygen samples can be used to
determine the site of entry into the right side of the heart and the size of a left-to-right
intracardiac shunt at atrial, ventricular, or pulmonary artery levels in
patients with congenital heart disease. Oxygen values from the pulmonary artery
are used with other data to calculate the pulmonary blood flow with thermodilution
and balloon-tipped catheters. Measurement of pressures through the catheter using
external pressure transducers allows determination of the phasic form of the pressure
in any location. Pressures recorded as a catheter traverses a valve permit an evaluation
of the site and degree of valvular stenosis.
Special sensors
at the tip of a catheter have been designed for the detection and recording of intracardiac
ECGs and pressures.
Complications
Brief arrhythmias,
vasovagal episodes, and minor phlebitis may be observed in patients undergoing catheterization.
More serious complications are rare.
 |
| LEFT-SIDED HEART CATHETERIZATION |
LEFT-SIDED HEART CATHETERIZATION
Technique
The aim of left-sided
heart catheterization is the study of conditions in the chambers and vessels
of the left side of the heart. In congenital heart disease the catheter may reach
the left side of the heart from a right-sided heart chamber, passing through an
atrial septal defect or a patent ductus arteriosus.
More often the left
side of the heart is approached by retrograde passage of the catheter from
its point of insertion into a peripheral artery, most commonly by
percutaneous technic. This technique was designed by Seldinger (see Plate 2-9). The catheter is manipulated under fluoroscopic
control in a retrograde direction using the Seldinger technique through the artery
to the aorta and frequently across the aortic valve into the left ventricle. Entry
into the left atrium retrograde through the mitral valve is possible but not typically
used. Approach to the left atrium can also be accomplished using a transseptal technique
by passage of the transseptal catheter and transseptal needle from a right
femoral vein to the right atrium and across the atrial septum at the level of
the fossa ovalis. The catheter can then be advanced into the left ventricle. Direct
percutaneous needle puncture of the LV apex may be done to reach the left ventricle
in special circumstances, such as LV pressure measuremen in patients with mechanical
aortic and mitral valves.
Diagnostic
Procedures
Sampling and pressure
measurements for left-sided heart catheterization do not differ from right-sided
procedures. Valvular abnormalities can be estimated using simultaneous pressure
measurements on both sides of the valve.
Complications
Arrhythmias, the
most common complication of leftsided catheterization, usually respond to simple
catheter withdrawal, although rarely may require therapy. Other complications include
arterial spasm and the rare dissection or occlusion of the artery. Perforations
of the walls of an artery or the aorta, a heart chamber, or a coronary artery also
can occur rarely. Fluid should never be forced through a catheter from which blood
cannot be withdrawn, particularly if the catheter is in the ascending aorta; a clot
can b expressed and embolize peripherally to the brain.
 |
| NORMAL SATURATIONS (O2) AND PRESSURE |
CARDIAC OUTPUT: THERMODILUTION TECHNIQUE
A balloon-tipped
pulmonary artery catheter (Swan-Ganz catheter) with a thermistor at the tip (introduced
in 1970) floats into the pulmonary artery from the right ventricle as an embolus
when the balloon is inflated. The balloon occludes the distal pulmonary branches,
and a pressure similar to the PCWP can be measured. When deflated, the catheter
measures pulmonary artery pressure, and the thermistor measures a thermodilution
curve after injection of 10 mL of cold saline or glucose into the right atrium.
The cardiac output can be calcu- lated from the measured thermodilution curve.
When the cardiac output is low, the temperature change from right to distal pulmonary
artery changes little. When the cardiac output is high, the temperature change is
large. Thus the degree of change in temperature is directly proportional to the
cardiac output.
NORMAL OXYGEN
SATURATIONS AND PRESSURE
In the venae cavae,
right atrium, right ventricle, and pulmonary arteries, oxygen saturation (SO2)
is normally close to 75% (see Plate 2-10). Small, phasic
variations in So2 of blood sampled from the right-sided heart
chambers can be measured. The variation is maximal in the right atrium, where contributions
of blood from the renal veins (with a relatively high So2), from the
hepatic veins (with relatively low So2), from the coronary sinus
(with very low So2), and from the lower inferior vena cava and superior
vena cava (with intermediate So2), meet and start mixing. The mixing
is probably complete by the time the blood reaches the pulmonary artery. In the
pulmonary wedge position, 97% to 99% saturated blood can be withdrawn through
the wedged catheter, approximating the values of pulmonary venous blood. Blood leaving
the pulmonary capillary bed is at least 97% saturated. Blood entering the left
atrium is slightly less saturated because of its admixture with blood passing
through pulmonary arteriovenous and other small shunts.
NORMAL INTRACARDIAC
PRESSURES
Atrial and
Wedge Pressures
The phasic pressures
in the right atrium, the left atrium, and the pulmonary artery wedge position (essentially
a slightly delayed left atrial pressure) share the same characteristics, with small
differences in the amplitude and timing of the phasic features. In normal sinus
rhythm the pressure pulse in these chambers is characterized by an a wave
produced by the atrial contraction that begins with completion of the atrial P wave
in the electrocar-diogram (ECG). After a brief delay, the P wave is followed by
the QRS signaling the depolarization of the ventricular myocardium. Immediately
after depolarization, ventricular contraction begins. The A-V valves close, and
the c waves in the atrial pressure curves are produced by changes in the
dimensions of the atria and by bulging of the valves into the atria secondary to
ventricular contraction. After the c wave, pressure decreases to a low value
(the x descent) in response to further atrial volume changes during continued
ventricular contraction. During the remainder of systole, continuous venous
inflow produces an increase in pressure, the v wave). The peaks of the v
waves coincide with the opening of the mitral and tricuspid valves. A
pressure decrease in the atria (the y descent) accompanies the transfer of
blood from the atria into the ventricles.
Except for the peak
systolic pressure in the left ventricle being approximately five times greater than
that in the right, the phasic pressures in the left and right ventricles are similar
in contour. The ventricles begin to contract approximately 60 milliseconds after
the QRS in the ECG, with the right preceding the left. This action is
associated with closure of the A-V valves, resulting in elevated ventricular pressures.
During the subsequent period of sequential myocardial contraction, lasting 10
msec and 40 msec for the right and left ventricles, respectively, there are no volume
changes, the period of isovolumic contraction.
When the ventricular
pressures exceed the end-diastolic pressures in the pulmonary artery and
aorta, the semilunar valves open and ejection begins. During the ejection period,
the right ventricle and pulmonary artery and the left ventricle and aorta have the
same phasic pressures until, systole being completed, the semilunar valves close
and the pressures begin to drop in the ventricles. This is followed by the
brief period of isovolumic relaxation.
As soon as the ventricular
pressures fall below the pressures in the atria, the A-V valves open; and diastole
starts and proceeds with venous filling of the common ventricular and atrial chambers,
leading to superposable pressures in the atria and ventricles.
During ejection
the ventricular pressures and the pressures in the aorta or pulmonary artery
are identical and are characterized by a smooth rise to a peak, then a steady fall
to the dicrotic notch, signaling the closure of the aortic and pulmonary valves.
This is followed by a steady decrease in pressure as a “runoff” of blood from
the arterial system into the venous system occurs through the capillary beds. This
is abruptly terminated by the next ejection.
EXAMPLES OF O2 AND PRESSURE FINDINGS AND PRESSURE TRACINGS IN HEART DISEASES
ABNORMAL OXYGEN AND PRESSURE
FINDINGS
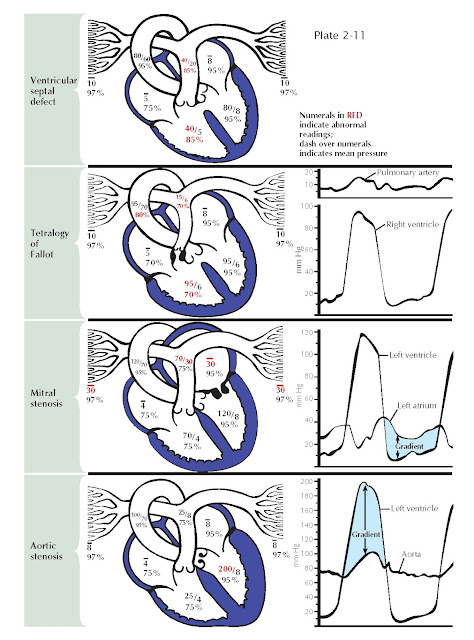
In ventricular septal
defect a shunt of 95% saturated blood is ejected during systole by the left ventricle
through the defect into the right ventricle, under
the influence of the normally occurring pressure difference between the two ventricles
(see Plate 2-11). There the shunted blood contaminates
the less-saturated mixedvenous blood. Thus an increased volume of blood with a greater-than-normal
So2 (85%) flows into the pulmonary artery. In the majority of
cases the volume of blood shunted depends on the systolic pressure difference
between the two ventricles and on size of the defect. The increased So2
of the blood in the pulmonary artery is in direct proportion to the volume of the
shunt. The pressures in the pulmonary artery and right ventricle are usually elevated
because of the increased pulmonary vascular resistance, which is secondary to the
failure of neonatal involution to take place in the normal prenatal medial hypertrophy
of the small arteries. The pressures may be greatly elevated by subsequent intimal
and other pathologic changes. Eventually, after development of very high RV pressures,
the shunt may be reversed, and desaturated blood may flow from the right to the
left ventricle and the systemic arteries.
Tetralogy
of Fallot
The basic abnormalities
in the tetralogy of Fallot are pulmonary stenosis (valvular or infundibular) interventricular septal defect, disproportion in the diameter between and usually some displacement
of the aorta and pulmonary artery, with secondary RV hypertrophy (see Plate 2-11). Because of the pulmonary stenosis, which
significantly increases normal outflow resistance, RV hypertension may reach systemic
levels. This results in a shunt of unsaturated blood through the defect, with a
mild reduction in So2 in the left ventricle and a greater reduction in the aorta and systemic
arteries. The latter causes the cyanosis characteristic of these patients. The
greatly reduced pulmonary blood flow reaches full saturation in the lungs. Systolic
pressure in the right ventricle reaches the level of the aortic pressure. Distal
to the pulmonary stenosis, however, the pressures are lower than normal, and the
pressure contour is often distorted.
The resistance to diastolic flow from left atrium to left
ventricle after narrowing of the mitral valve increases left atrial (LA) pressures
and eventually reduces LV flow (see Plate 2-11). A pressure gradient across the
mitral valve throughout diastole can be demonstrated by simultaneous PCWP measurements
or direct LA and LV pressure measurements. This gradient is inversely proportional
to the square of the cross-sectional area of the valve orifice and is directly proportional
to the square of the volume flow. The gradient is greater with increases in the
degree of stenosis and during exercise. The LA hypertension is accompanied by pulmonary
venous hypertension, which results in pulmonary hypertension and RV hypertension,
increased RV work, and hypertrophy. Diastolic pressures in the pulmonary artery
and left atrium are identical until pulmonary vascular resistance is increased because
of pathologic changes in the vascular bed, resulting in a gradient between the two
pressures. Acute bouts of LA hypertension lead to pulmonary edema, whereas chronic
pulmonary artery hypertension may eventually cause RV failure.
Aortic Stenosis
In aortic stenosis,
obstruction to the ejection of blood from the ventricle into the aorta, caused by
subvalvular, valvular, or supravalvular stenosis, results in abnormally high pressure
in the left ventricle and abnormally low pressure in the aorta and thus a systolic
pressure gradient across the valve. Progressive obstruction to LV outflow magnifies
these effects and leads to LV hypertrophy and eventually acute or chronic LV systolic
and diastolic failure (see Plate 2-11).
NORMAL CARDIAC BLOOD FLOW DURING INSPIRATION AND EXPIRATION
EFFECTS OF INSPIRATION
AND EXPIRATION ON INTRACARDIAC PRESSURES AND FLOW
During inspiration, systolic blood pressure decreases and pulse rate increases slightly, because the intrathoracic pressure becomes more negative relative to atmospheric pressure. Systemic venous return increases, more blood flows into the right side of the heart, and pulmonary vasculature compliance increases (see Plate 2-12). This results in pooling of blood in the lungs and a decrease in pulmonary venous return, reducing flow to the left side of the heart. The reduced left-sided heart filling leads to a decreased stroke volume and systolic blood pressure. The decrease in systolic blood pressure leads to a faster heart rate because of the baroreceptor reflex, which stimulates sympathetic outflow to the heart. These changes are reversed with expiration.