Development and Developmental Disorders of the Hypothalamus
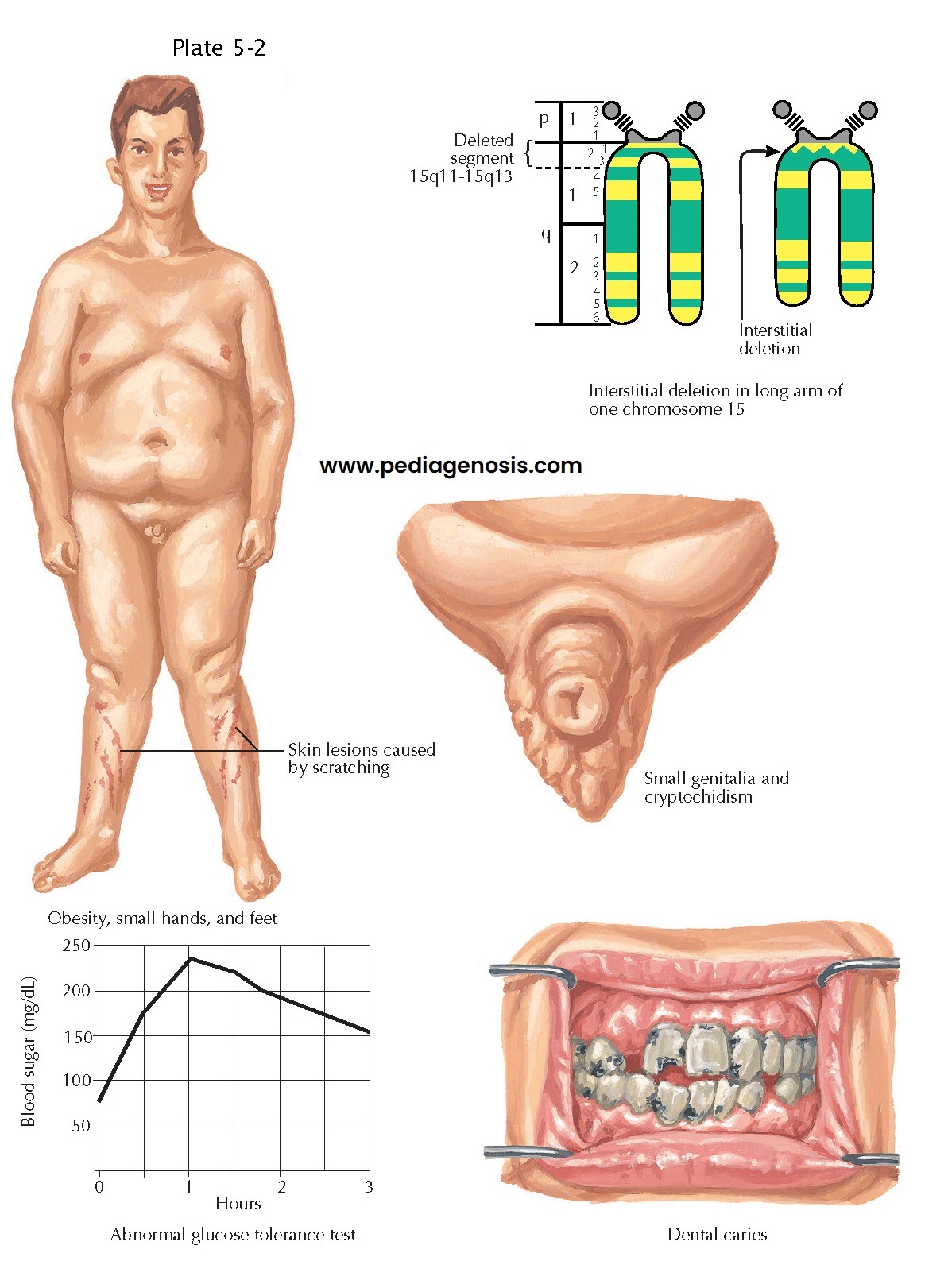 |
| CYTOGENETIC DISEASE: PRADER-WILLI SYNDROME |
The hypothalamus in mammals arises as a part of the ventral diencephalon and the adjacent telencephalon, and its embryologic origins are intimately related to those of the optic chiasm and tracts and to the pituitary gland. Thus disorders that affect the hypothalamus fre- quently manifest with signs and symptoms resulting from dysfunction of neighboring, developmentally related structures. The developing neural tube is divided into three primary regions: forebrain, midbrain, and hindbrain. The forebrain is further subdivided into the telencephalon, which gives rise to the cerebral cortex and basal ganglia, and the diencephalon, from which the thalamus and hypothalamus are derived. The hypothalamus develops from the anterior portion of the diencephalon in a series of steps that involve the activation of suites of transcription factors, which determine the fates of the developing cell populations.
First, the
prechordal mesoderm that underlies the developing neural tube secretes sonic
hedgehog (Shh) that induces the normal patterning of the anterior midline of
the brain, including the formation of the hypothalamus and the separation of
the optic system. Abnormal mesodermal induction occurs with mutations that
affect Shh signaling and can result in one of the most common human brain
malformations, holoprosencephaly, which manifests with a spectrum of failed
division of the midline structures of the brain. In its most severe form,
holoprosencephaly results in cyclopia and complete or partial loss of the
hypothalamus, which is not compatible with life. In its more mild forms,
holoprosencephaly can manifest with endocrine abnormalities because of
defective development of the hypothalamic-pituitary system. After initial
patterning by Shh-mediated induction, hypothalamic precursor cells
proliferate before exiting the cell cycle and undergo terminal differentiation
into the many cells types that comprise the hypothalamus’ compact, yet complex
structure. Finally, the developing neurons express unique combinations of transcription
factors, such as Nkx and Lhx family members, and Sim1, and Six3. Deletions of
individual transcription factors have pro-found effects upon development of
specific hypotha- lamic nuclei.
Terminal
differentiation of the hypothalamic nuclei requires the combined action of
“codes” of transcription factors that, when expressed with anatomically
restricted and developmentally timed precision, give rise to the regional
complexity of the hypothalamus. Although still poorly understood, rare genetic
mutations have been identified in humans and tested in animal models that
demonstrate that dysfunction of specific genes results in loss of specific
hypothalamic neurons and corresponding phenotypes. For example, the
Prader-Willi syndrome, which manifests as morbid obesity, hypersomnolence,
hypogonadism, and intellectual disability, is caused by a deletion of the
paternally inherited chromosome 15q11. This genomic region contains several
genes implicated in the normal development of the paraventricular nucleus, a cell
group with critical integrative functions in feeding and responses to stress
(see later).
The relationship of the hypothalamus and pituitary gland has its embryologic origins as an anatomic juxta-position between the anterior diencephalon and the ectodermally derived Rathke’s pouch, from which portions of the ventral pituitary are derived. Thus both the hypothalamus and pituitary are patterned by similar signaling pathways, and dysfunction in these systems may disrupt the development and function of both structures. Craniopharyngiomas are the most common non-neural intracranial tumors in childhood and derive from the remnants of Rathke’s pouch. Clinical presentation includes optic, pituitary, and/or hypothalamic symptoms, including obesity, hypopit itarism, and sleep and circadian rhythm dysfunction.




