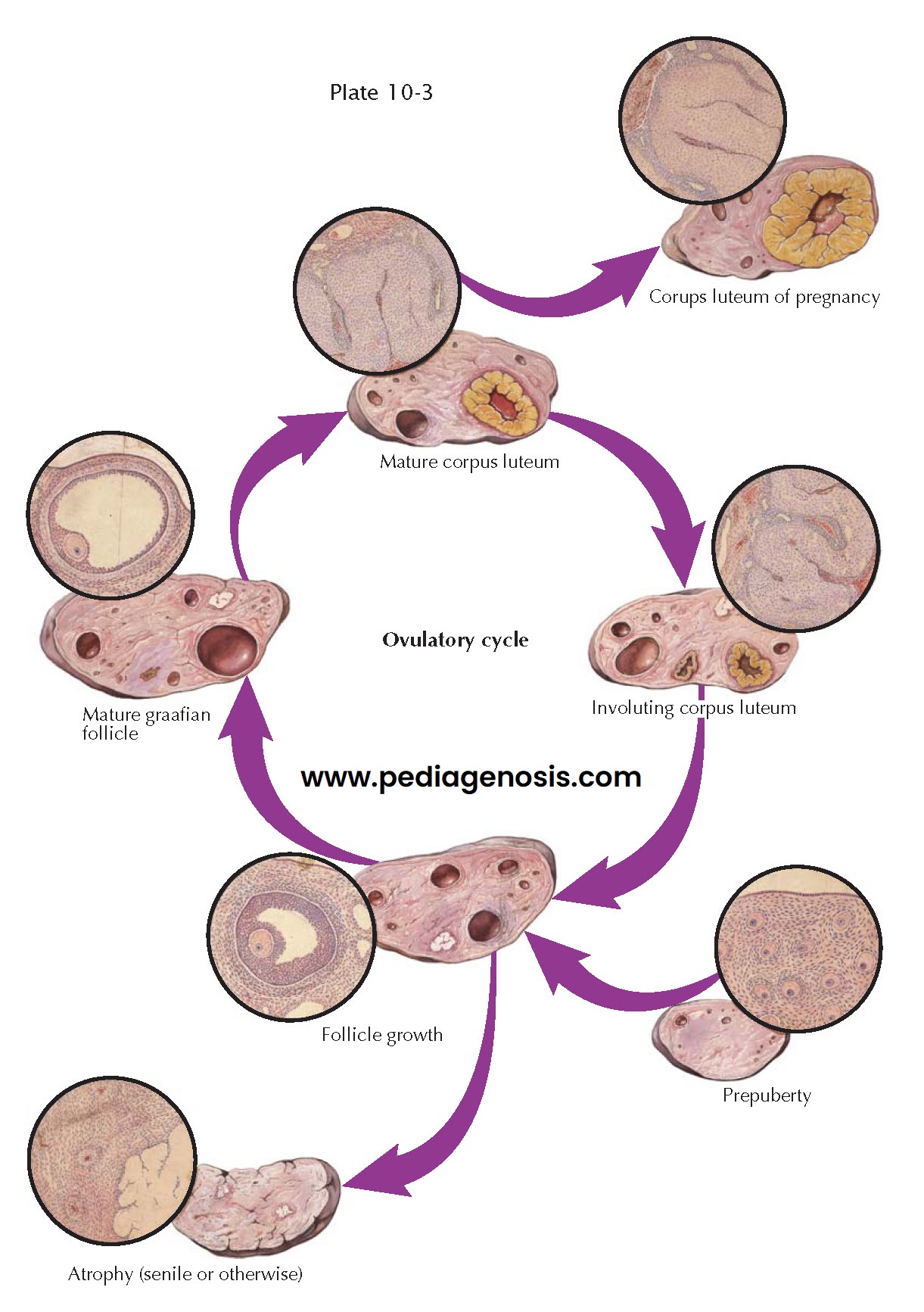
OVARIAN CYCLE
Although follicle growth is relatively even, it is slow for 10 to 12 days, during which time the granulosa layer thickens and the antrum becomes distended by increasing amounts of estrogen-rich liquor folliculi. After the follicles have reached a diameter of about 0.5 cm, they start their migration outward toward the surface, and about 12 days after the start of this cycle of growth, one follicle “gains ascendancy” in one of the ovaries, reach- ing a diameter of 1.5 to 2 cm in a short time. Simultaneously, it begins to protrude from the ovarian surface. This surge of development is accompanied by a similarly sudden wave of atretic processes, which wipes out all lesser developing follicles in both ovaries. Because the granulosa is the first layer to degenerate in this change leading to atresia, it is probable that the theca is responsible for transient continuing secretion.
Whether follicle migration is accomplished by hor- monal or enzymatic
reactions or merely by the physical forces involved in a rapidly expanding
cystic structure enclosed in a tough, fibrous stroma by a relatively inelastic
tunica is not known. During this development under the influence of the
luteinizing hormone (LH) surge, the ovum undergoes the first maturation division
and the first polar body is extruded, reducing the number of chromosomes from 46
to 23. The granulosa cells become widely separated by edema, thus loosening the
egg and its surrounding cumulus for detachment from the inside of the follicle
cavity. Finally, through compression of the capillary net at the weakest point
of the follicle wall near the surface, a relatively avascular area is produced,
and through this area a break occurs. Intrafollicular pressure forces the egg,
cumulus, and some detached granulosa cells with the liquor folliculi out into
the peritoneal cavity.
Bleeding to some degree from the break in the richly vascular theca
interna always attends ovulation. Usually, the rupture point is rapidly sealed
off, though in patients with compromised clotting ability, this can result in
hemorrhage. The cavity of the follicle is filled with blood-containing fluid—the
so-called corpus haemor-rhagicum—and an immediate differentiation of cells sets
in, which spreads inward from the granulosal remnants. These lipid-and
pigment-containing cells—the lutein cells—grow on a network of capillaries from
the theca interna. This process causes an appearance of infolding as the walls
of the former follicle thicken and encroach more and more on the
fibrin-containing, blood-filled cavity ultimately representing the mature corpus
luteum. When pregnancy occurs, this development continues under the influence of
human chorionic gonadotropin and increases until the bright-yellow body may
make up as much as one half the total volume of the ovary—the corpus luteum of
pregnancy. Some time after the second month of gestation, a slow process of
regression of the corpus luteum occurs and is accompanied by the
luteal–placental shift, where the placenta becomes the primary organ of
hormonogenesis for the remainder of pregnancy.
If conception does not take place, the fate of the corpus luteum is
involution. The crenated, yellow margin shrinks relatively rapidly, and the
lutein cells degenerate into amorphous, hyaline masses held together by strands
of connective tissue. The yellow color is in most part lost. The shrunken
convolutions of hyalinized material are known as a corpus albicans. It is
during this regressive phase that the decrease of estrogen and progesterone
production prompts a new output of pituitary gonadotropins, producing
stimulation of a new crop of ovarian follicles, and a new ovula- tory growth
cycle is once more initiated.
The senile ovary is a shrunken and puckered organ, containing few if any follicles, and made up for the most part of old corpora albicantia and corpora atretica. In the menopause, the senile ovary continues to produce hormones, albeit at lower levels and with a more androgenic profile.