Shock
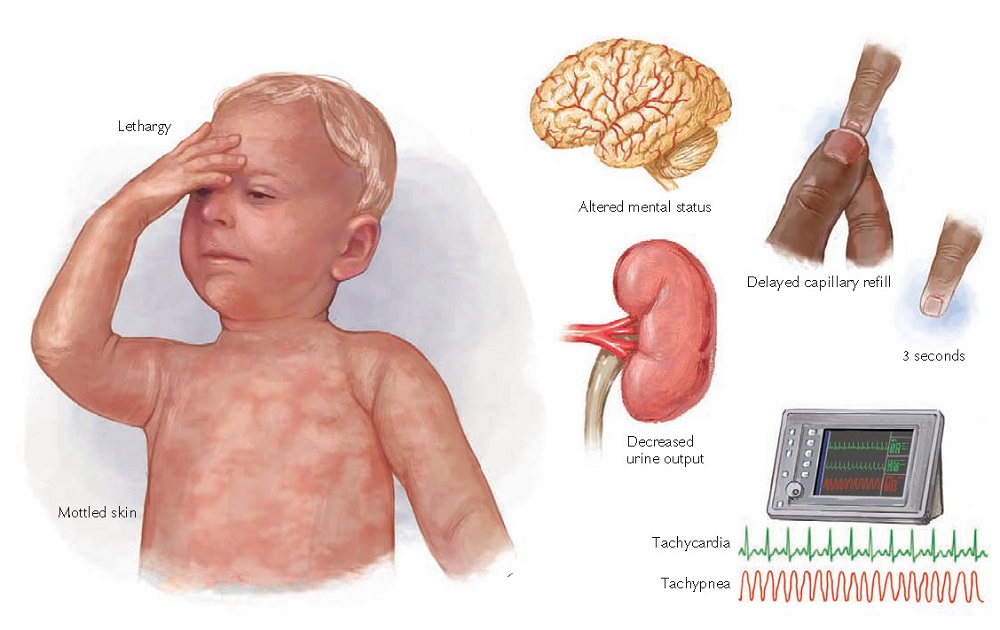 |
Figure 2-1 Clinical manifestations of shock.
Shock is an acute clinical syndrome of circulatory dysfunction in which there is failure to deliver sufficient oxygen and substrate to meet metabolic demand. All practitioners who care for children must understand and identify shock promptly to initiate an effective treatment plan. This, in turn, can help prevent the progression and poor outcomes that characterize the natural clinical course of shock. The goal is to prevent end-organ damage; failure of multiple organ systems; and, ultimately, death.
ETIOLOGY AND PATHOGENESIS
Normal circulatory function is maintained by the interplay between the heart and blood flow with the purpose of delivering oxygen and nutrients to the tissues. Cardiac output is calculated by multiplying the stroke volume (volume of blood ejected by the left ventricle in a single beat) by the heart rate (ejection cycles per minute). Stroke volume is dependent on the filling volume of the ventricle (preload), resistance against which the heart is pumping blood (afterload), and myocardial contractility. During childhood, the heart rate is faster, and the stroke volume is smaller than during adulthood. In children, increasing the heart rate is the primary means to increase the cardiac output. Shock develops as the result of conditions that cause decreased intravascular volume, abnormal distribution of intravascular volume, or impaired cardiovascular function. Children effectively compensate for circulatory insufficiency by increasing their heart rate, systemic vascular resistance (SVR), and venous tone. Children can therefore maintain normal blood pressures despite significantly compromised tissue perfusion. Thus, in pediatric patients, it is especially important to recognize that hypotension is not part of the definition of shock.
The clinical manifestations of shock
can be directly related to the abnormalities seen on the tissue, cellular, and
biochemical levels. Microcirculatory dysfunction; tissue ischemia; and release
of biochemical, vasoactive, and inflammatory mediators are all part of the
spectrum of pathophysiologic aberrations seen in shock. Poor perfusion of vital
organs results in impaired function. For example, inadequate perfusion of the
brain and kidneys results in depressed mental status and decreased urine
output, respectively. As poorly perfused cells switch to anaerobic metabolism
to generate energy, lactic acid accumulates resulting in a metabolic acidosis
that further interferes with cell function. Hypoperfusion also initiates
inflammatory events, such as the activation of neutrophils and release of
cytokines, that cause cell damage and microischemia.
The prevalence of causes of shock
varies by patient age, as well as region of the world. Hypovolemic shock from
diarrheal illness is the leading cause of pediatric mortality worldwide, but is
very rare in the United States. Congenital lesions (including heart disease)
and complications of prematurity are most common in neonates and infants.
Malignant neoplasms (for whom infectious complications are prevalent),
infectious causes, and unintentional injuries are
more common in older children and young adolescents. Injury, homicide, and
suicide become more prevalent in older adolescents.
Compensated (Early) Shock
In compensated shock, homeostatic
mechanisms have temporarily balanced metabolic supply and demand. In this
state, the systolic blood pressure is normal in the presence of inadequate
tissue perfusion. The earliest symptoms of shock result from an effort to
maintain cardiac output and perfusion of vital organs (heart, brain, and
kidneys). The body’s initial mechanism to maintain cardiac output and
compensate for low stroke volume is to increase heart rate (tachycardia). Other
compensatory mechanisms include increasing SVR, cardiac contractility, and
venous tone. As shock continues, the early compensatory mechanisms fail to meet
the metabolic demands of the tissues, and uncompensated shock ensues. Here, as
the microcirculation is affected, the child shows signs of brain, kidney, and
cardiovascular compromise.
Uncompensated (Late) Shock
Uncompensated shock occurs when
attempts to maintain blood pressure and perfusion are no longer successful,
resulting in hypotension. When hypotension develops, the child’s condition may
deteriorate rapidly to cardiovascular collapse and subsequent cardiac arrest.
Eventually, the child in uncompensated shock develops multiple organ
dysfunction syndrome (MODS) secondary to ongoing shock and exaggerated
inflammatory responses. Irreversible shock implies irreversible damage to vital
organs resulting in death, regardless of therapy.
CLASSIFICATION OF SHOCK
Hypovolemic Shock
The most common type of shock in
children is hypovolemic shock. Hypovolemia is defined as a decrease in
circulating blood volume. The most common cause of hypovolemic shock is fluid
loss associated with diarrhea and vomiting. Other causes include blood losses
(e.g., trauma and gastrointestinal disorders), plasma losses (peritonitis,
hypoproteinemia, burns), and water losses (osmotic diuresis, heatstroke). In
hypovolemic shock, preload is decreased, SVR may be increased as a compensatory
mechanism, and cardiac contractility is typically normal or may be increased.
Distributive Shock
Distributive shock is the result of
abnormal distribution of blood volume (i.e., poor flow to the splanchnic
circulation with excessive flow to the skin) caused by vasodilatation from
changes in vasomotor tone and peripheral pooling of
blood, resulting in inadequate tissue perfusion. SVR can be low, producing
increased blood flow to the skin that keeps the extremities warm (warm shock),
as well as a widened pulse pressure and bounding peripheral pulses. Conversely,
SVR may be increased, resulting in decreased blood flow to the skin, resulting
in cool extremities, with a narrowed pulse pressure and weak pulses (cold
shock). Generally, cardiac output is normal or increased. Distributive shock
commonly occurs in anaphylaxis; central nervous system or spinal injuries; drug
ingestions; and most commonly in children, sepsis.
Cardiogenic Shock
Cardiogenic shock results from
myocardial dysfunction and can usually be distinguished from other forms of
shock because of associated signs of congestive heart failure (i.e., rales,
gallop rhythm, hepatomegaly, jugular venous distension). Pump failure,
arrhythmias, and congenital heart disease may all contribute to the inadequate
perfusion seen in cardiogenic shock. A patient may exhibit tachycardia,
increased SVR, and signs of decreased cardiac output as a result of a decrease
in myocardial contractility. Causes of cardiogenic shock in children include
viral myocarditis, arrhythmias, drug ingestions, complications of cardiac
surgery, trauma, metabolic derangements, and congenital heart disease.
Cardiogenic shock can also occur with obstruction of blood flow, as seen with a
tension pneumothorax, massive pulmonary embolism, or critical coarctation of
the aorta or other obstructive vascular lesions. A patient may also show
evidence of both intrinsic cardiac disease and obstruction of blood flow with a
cardiac tamponade or ductal-dependent congenital abnormality. It is important
to recognize that infants that present with shock caused by a ductal-dependent
cardiac lesion require blood flow through the ductus arteriosus to maintain
adequate oxygen delivery.
Neurogenic Shock
Neurogenic shock may occur in the
setting of pediatric trauma. Spinal cord injury may produce hypotension caused
by a loss of sympathetic tone. The classic picture of neurogenic shock is
hypotension without tachycardia or cutaneous vasoconstriction. The pulse
pressure is usually widened. Patients sustaining spinal injuries often have
concurrent torso trauma. Therefore, patients with known or suspected neurogenic
shock should be treated initially for hypovolemia.
Septic Shock
Sepsis is defined as the presence of
the systemic inflammatory response syndrome (SIRS) caused by a presumed or
confirmed infection (Box 2-1). Sepsis may occur because of bacterial, viral,
fungal, or parasitic infections. Septic shock is defined as sepsis and
cardiovascular dysfunction. Classifying septic shock may be difficult because
of the developmental variability in physiologic response to sepsis. A clinical
picture consistent with hypovolemic, distributive, or cardiogenic shock may be
present in a child with sepsis. Additionally, studies have demonstrated that
the cardiovascular pathophysiology of children with sepsis can evolve over time, and the adjustment of
hemodynamic therapy is commonly necessary.
CLINICAL MANIFESTATIONS AND
EVALUATION
Shock remains a clinical diagnosis
(Figure 2-1). Early recognition of the clinical signs of shock (including
familiarity with normal ranges for vital signs by age; see Chapter 1) should
lead to directed management. An accurate history should be obtained from the
family and, if possible, the child, simultaneously with treatment initiation.
A history of fluid loss, as with a
gastrointestinal bleed, gastroenteritis, or diabetic ketoacidosis, is
consistent with hypovolemic shock. A detailed trauma history is useful because
an injured child may have hypovolemic shock from hemorrhage (i.e., with blunt
abdominal trauma), neurogenic shock with spinal cord injury, or obstructive
shock from tension pneumo- thorax. A child who has had fever or is
immunocompromised may have features consistent with septic shock. Exposure to
an allergen, such as a food or an insect bite, could suggest distributive shock
caused by anaphylaxis. A history of ingestion or medications should always be
included when speaking to the family because shock may be attributable to toxin
exposure. Patients with underlying heart disease may present in cardiogenic
shock. Patients with a history of adrenal insufficiency (i.e., chronic steroid
therapy, congenital adrenal hyperplasia, or hypopituitarism) can present with
adrenal crisis and shock.
A complete physical examination
should be performed, including vital signs and pulse oximetry. When a child
presents in shock, it is sometimes difficult to obtain an accurate weight,
which can be essential for determining fluid requirements and medication doses.
If the patient’s weight cannot be measured, one may be estimated using a
length-based tape system (e.g., the Broselow tape) or the child’s age.
Children in shock tend to be
tachypneic, as well as tachycardic. Blood pressure should be monitored closely.
Remember, children
with shock may have normal blood pressures. Narrow pulse pressure may occur as
a result of a compensatory increase in SVR, as in hypovolemic or cardiogenic
shock. Widening of the pulse pressure can be seen as the result of decreased
SVR, as can occur with distributive shock. The child’s temperature should also
be measured because fever—or in young infants, hypothermia—may suggest septic
shock.
When first examining an ill child,
one should do a rapid assessment of mental status. Change in the level of
consciousness of a child may indicate decreased cerebral oxygenation or
perfusion. Signs of diminished perfusion to the brain include confusion,
irritability, lethargy, and agitation.
Examining the child’s skin is
another way to assess perfusion and the degree of shock. A child with normal
cardiorespiratory function should have warm and pink nailbeds, mucous
membranes, palms, and soles. As shock progresses and poor perfusion develops,
the skin may become cool, pale, or mottled. Capillary refill, although limited
by clinician variability as well as ambient temperature and the child’s body
temperature, can help to evaluate children in shock. Light pressure is applied
to blanch the fingernail bed. The pressure is released, and the amount of time
until color returns is measured. Normal is less than 2 seconds; volume
depletion or poor perfusion can increase this time to greater than 3 seconds.
The evaluation of a child with poor
perfusion and shock should always include an
assessment of pulses. This includes the rate, strength, and regularity of the
central and peripheral pulses. In healthy children,
the carotid, brachial, radial, femoral, dorsalis pedis, and posterior tibial
pulses are readily palpable. A rapid pulse is a nonspecific clinical sign of
distress. An irregular pulse is a warning of cardiac dysrhythmia. A weak pulse
raises the concern for shock and a severe hypovolemic state. An absence of
central pulses indicates ineffective or absent cardiac contractions and
signifies the need for immediate resuscitative action.
After the initial evaluation of
airway, breathing, and circulation (the ABCs), a complete physical examination
can help elucidate the type of shock. For example, central cyanosis, a gallop
rhythm, crackles on lung examination, hepatomegaly, or heart murmur may
indicate an underlying cardiac condition. Children with stridor, wheeze,
urticaria, or edema may have anaphylactic shock. Purpura or petechiae can be
seen in children with septic shock. Bruises and abrasions can be seen with
traumatic injury and may give a clue to underlying hemorrhagic shock.
 |
Figure 2-2 Algorithm for management of pediatric septic
shock.
MANAGEMENT
General Principles
Early recognition of compensated
shock is critical to ensuring appropriate and expedient therapy. Initial therapy of shock is universal,
regardless of the cause of the shock state, with the goals of optimizing blood
oxygen content, improving cardiac output, reducing oxygen demand, and
correcting metabolic abnormalities (Figure
2-2). General principles of resuscitation should be applied immediately on
presentation to medical care (see Chapter 1). Ultimately, after initial
management has commenced, correction of the underlying cause is essential
(e.g., stopping blood loss in hemorrhagic shock, antibiotics for shock caused
by bacterial infection).
Immediate attention to the ABCs is
mandatory. Maintenance of a patent airway with positioning or endotracheal
intubation should be performed immediately if airway compromise is present.
Hypoxemia should be corrected without delay; all patients with compromised
perfusion should receive supple- mental oxygen at 100% FiO2
(fraction of inspired oxygen). Insufficient respiratory effort should be
addressed with positive-pressure ventilation.
Imminently life-threatening causes
of shock should be identified and corrected. For example, a child with upper
airway obstruction from anaphylaxis should receive epinephrine. If a child has
severe respiratory distress, asymmetric breath sounds, and poor perfusion, a
tension pneumothorax might need to be decompressed.
Vascular access is indicated in all
cases. If possible, large-bore intravenous (IV) catheters should be inserted in
peripheral veins. If an IV line is unable to be placed promptly, intraosseous
(IO) cannulation should be performed. IV fluid boluses of 20 mL/kg of isotonic saline should be given
rapidly and repeated as needed with reassessment occurring simultaneously.
Rapid fluid administration should be actively performed using either a pressure
bag or a push–pull system rather than using passive gravity flow for
administration. IV fluids should be given with care in cardiogenic shock, so as
to not worsen associated pulmonary edema.
Adequate hemoglobin is essential for
optimal oxygen carrying capacity. Thus, in cases of hemorrhagic shock,
O-negative packed red blood cells should be rapidly administered early in the
resuscitation. Even in cases of nontraumatic shock, children who have cyanotic
heart disease or neonates may require less fluid and higher hematocrit
percentages to ensure adequate oxygen carrying capacity. Life-threatening
metabolic abnormalities should be identified and corrected early. Hypoglycemia
should be treated with 0.5 to 1 g/kg of IV dextrose. Hypocalcemia (especially
decreased ionized calcium) is common in septic shock and can occur as acidosis
resolves; it should be corrected with either calcium gluconate or calcium
chloride.
Circulatory Support
Depending on the cause, patients in shock may require large volumes of fluid as well as vasoactive medications. Clinical studies of septic shock in children have demonstrated an association between higher volumes of fluid administration and survival. Current guidelines for septic shock recommend up to and over 60 mL/kg in the first 15 to 60 minutes. Every hour that goes by without implementation of this therapy is associated with a 1.5-fold increase in mortality. A retrospective chart review of 90 children with septic shock showed that those who received less than 20 mL/kg of fluid within the first hour had a mortality rate of 73%. Early fluid resuscitation was associated with a threefold reduction in the odds of death.
Vasoactive agents help improve
cardiac output through their effects on myocardial contractility, heart rate,
and vascular tone.
These drugs target at least three
types of receptors. The β1- receptors mediate inotropic (contractility), chronotropic
(rate), and dromotropic (increased conduction velocity)
activity. The β2-receptors
mediate vasodilatation and smooth muscle relaxation in blood vessels and
bronchial tree. The α-receptors
mediate arteriole constriction systemically and bronchial muscle constriction. The dopaminergic receptors mediate
smooth muscle relaxation and increase renal blood flow and sodium excretion.
Table 2-1 outlines commonly used
vasoactive agents, their targeted receptors, and their hemodynamic effects.
Data are varied on the choice of initial agent; current recommendations from
the American College of Critical Care Medicine state that dopamine,
epinephrine, or norepinephrine may be appropriate first-line therapy for septic
shock, provided they are administered through a central venous catheter.
Dopamine or low-dose epinephrine may be given via a peripheral vein while
central venous access is obtained.
Special Circumstances: Shock in
Neonates
Neonatal physiology poses specific
challenges regarding the management of shock. As mentioned previously, optimal
oxygen-carrying capacity may demand a higher hematocrit in a newborn. Hypoxemia
may occur more readily because of the presence of smaller and fewer alveoli,
absent collateral channels of ventilation, and poor chest wall compliance;
uncorrected hypoxemia may result in bradycardia in neonates. Additionally, myocardial performance is more drastically
affected by acidosis and hypocalcemia in neonates.
Prompt correction of hypoxemia, acidosis, and hypocalcemia is essential.
Neonates are also more prone to hypoglycemia, which should be looked for and
treated appropriately.
When faced with a neonate with
shock, early consideration should be given to ductal-dependent congenital heart
disease. Lesions marked by ductal-dependent systemic blood flow, such as aortic
stenosis, hypoplastic left heart syndrome, coarctation of the aorta, and
interrupted aortic arch, may present as shock in the neonatal period (see
Chapter 44). Infants with these lesions depend on blood flow from the pulmonary
artery across the ductus arteriosus into the aorta for perfusion of all or part
of the systemic circulation. Although this is deoxygenated blood, the oxygen
content is sufficient to meet the metabolic demands of the tissues. Therefore,
when the ductus arteriosus closes, circulatory failure and tissue hypoxia occur.
Prostaglandin E1 (PGE1 or
alprostadil) is the definitive initial therapy for neonates with
ductal-dependent congenital heart disease who have not yet undergone surgical
palliation or correction. An infusion at 0.1 µg/kg/min is required to reopen a
closing ductus arteriosus. Side effects of prostaglandin include flushing,
hypotension, pyrexia, bradycardia, seizures, and apnea. Emergent evaluation by
a pediatric cardiologist should be pursued, but initiation of PGE1 therapy
should not be delayed pending the evaluation.
FUTURE DIRECTIONS
Current research in shock in children is predominantly in the realm of septic shock. Goal-directed therapy of septic shock, an established concept in adults, is less well investigated in children and will continue to be an important topic in future investigations. Diagnostic laboratory studies, such as serum lactate and B-type natriuretic peptide levels, may hold promise in early detection and ongoing monitoring of children with shock. Newer noninvasive techniques for cardiac output measurement (e.g., pulse contour waveform analysis, partial carbon dioxide rebreathing) have begun to be used successfully in children and may be applied more broadly in the years to come. Massive transfusion therapy in children with hemorrhagic shock is another area in which advances in therapy for adults are beginning to be investigated in pediatric patients. Additional advanced critical care therapies, such as steroid or thyroid hormone replacement, renal replacement therapy, newer hemodynamic agents (e.g., levosimendan), and extracorporeal circulatory support, have been studied in pediatric patients, but their exact role remains unclear.
































