Resuscitation
 |
| Figure 1-1 Pediatric and adult airway anatomy. |
Cardiopulmonary gency interventi or in respiratory extre PR) is the series of emergency interventions provided to a person who appears dead or in respiratory extremis, with the goal of restoring vital functions through optimization of cardiac output and tissue oxygen delivery. The two main components are external cardiac massage (chest compressions) and assisted respirations.
Most children who require CPR do not
survive. Those who do survive often have significant neurologic deficits from
the hypoxia and ischemia associated with the cardiopulmonary arrest. However, some
children do return to their premorbid function. This may be related to
recognition and treatment of impending cardiorespiratory failure, early
bystander CPR, or rapid correction of the life-threatening event. It is
difficult to predict which patients will have a return of spontaneous
circulation and ultimately survive. Therefore, high-quality CPR should be begun
immediately while more information is gathered to guide therapy.
The incidence of out-of-hospital
cardiopulmonary arrest is difficult to ascertain from the current literature.
However, data from multicenter registries support many widely held beliefs
about pediatric out-of-hospital arrests. The incidence of non-traumatic,
out-of-hospital cardiac arrest is highest for infants (children younger than 1
year of age). The most common cause in this age group is sudden infant death
syndrome (SIDS). The presenting rhythm is usually asystole, and survival to
discharge is rare (≈3%). For all other pediatric age groups, trauma
is the leading cause of death. Although asystole is the
most common arrest rhythm, ventricular tachycardia (VT) and
ventricular fibrillation (VF) do occur and are more common in older children,
especially adolescents. Survival in patients with ventricular dysrhythmias is
higher than in patients with cardiopulmonary arrest associated with rhythms
that are not responsive to cardio version or
defibrillation, such as asystole and pulseless electrical activity (PEA). For
nontraumatic arrests, survival even for older children remains low, about 9% in
those older than 1 year of age.
ETIOLOGY AND PATHOGENESIS
Cardiopulmonary arrest may result
from many causes. Sepsis, respiratory infection, pulmonary conditions,
drowning, SIDS, and injuries can lead to respiratory failure or shock. Without
effective intervention, cardiopulmonary failure (inadequate perfusion and
ineffective or absent respiration) ensues. Most pediatric arrests occur after
an initial respiratory arrest (rather than circulatory failure) and if
prolonged result in terminal rhythms of bradycardia; PEA; and, finally,
asystole. Patients in asystole likely have experienced a significant
hypoxic–ischemic insult.
CLINICAL PRESENTATION
The signs and symptoms of children
requiring immediate resuscitation are typically the result of failure of the
delivery of two vital substrates—oxygen and
glucose—to end organs (Table 1-1). Recognition of these manifestations through
a physical examination that focuses on airway, gas exchange, and cardiovascular
stability allows for rapid resuscitation of those who have failure of substrate
delivery and identification of those at risk for failure.
INITIAL ASSESSMENT
Evaluation of a critically ill or
injured child should begin with a general assessment. Physical
examination clues help the provider determine the extent of illness or injury
(i.e., whether the condition is life threatening or not) and identify systems
that require closer attention during the remainder of the assessment. The
Pediatric Assessment Triangle of the Pediatric Advanced Life Support (PALS)
course outlines the following components of the general assessment:
Appearance: muscle tone, interaction, consolability, look or
gaze, speech or cry
Work of breathing: increased work of breathing, decreased or absent
respiratory effort, or abnormal sounds
Circulation: abnormal skin color or bleeding
The initial assessment can be done
without laying hands on the patient and should take no more than several
seconds. If the patient’s condition is life threatening, additional support
should be recruited immediately. After these rapid initial impressions, the
clinician should aim to perform a swift yet careful primary assessment.
PRIMARY ASSESSEMENT
The primary assessment evaluates and
addresses vital functions in a systematic way with priority to systems that are
most crucial for sustaining life. Conveniently, the components of the primary
assessment can be remembered as the “ABCDEs”—airway, breathing, circulation,
disability, and exposure and environment. If a life-threatening
abnormality is identified at any point, the aberration should be addressed
before moving on in the assessment. With the publication of new guidelines for
cardiopulmonary resuscitation in 2010, it has been recommended that the
standard sequence of “ABC” be switched to “CAB” for patients who need
cardiopulmonary resuscitation. This recommendation is based on the recognition
that most cardiopulmonary arrests occur in adults and that even in children and
adolescents sudden arrest is more likely to be due to a cardiac arrhythmia.
Prompt institution of cardiac compressions to provide artificial circulation is
important to ensure the best possible outcome for individual patients. This is
especially important in out-of-hospital settings where early initiation of
bystander CPR has been shown to be one of the strongest predictors of survival
and good neurologic outcome. In medical settings the approach to resuscitation
may be individualized based on the clinical scenario.
Close attention to securing the
airway and providing artificial ventilation remain important but in the setting
of cardiopulmonary arrest chest compressions should not be delayed.
Airway
The patient’s airway is the first
priority. There are fundamental differences between the airway of a child and
that of an adult. The pediatric airway (Figure 1-1) is more anterior than the
adult airway, requiring less manipulation to bring the oral, pharyngeal, and
tracheal axes into alignment. In addition, the head-to- body proportion is
larger in infants than in adults, and thus extreme hyperextension of the neck
may exacerbate airway obstruction in younger children. The pediatric airway is
narrower, and the tongue is relatively large compared with the jaw, increasing
the risk of airway obstruction. The pediatric larynx is located more anteriorly
and cephalad than the adult larynx.
The provider should assess airway
patency using the “look, listen, and feel” approach.
The provider should look at the chest wall and listen to the
mouth and nose to detect whether there is evidence of air movement. Findings
suggesting airway obstruction include increased
respiratory effort with retractions, abnormal inspiratory sounds, or episodes
during which no airway or breath sounds are produced despite respiratory
effort. If a child is speaking, crying, or otherwise verbalizing, the airway is
intact. Attention should be paid to the quality of the sounds. A hoarse or
high-pitched cry should alert the provider to the possibility of airway
compromise without complete obstruction. Finally, the provider should feel for
air movement using a hand or cheek close to the patient’s mouth.
The most effective maneuvers for
opening an obstructed pediatric airway are the head tilt–chin lift or jaw
thrust techniques (Figure 1-2). In the head tilt–chin lift maneuver, the head
is tilted back slightly (without overextending), and the chin is lifted gently
with one finger on the bony prominence to avoid placing pressure on the soft
tissues of the neck. A roll or towel may be placed under the shoulders to
maintain the position.
If there is a risk of a neck injury,
it is critical to stabilize the cervical spine during evaluation of the airway
and avoid extending the neck. Manual cervical spine stabilization is
accomplished by holding the head in the midline position while applying gentle
cephalad traction. A cervical collar may be applied, taking care to size the
collar appropriately. It is important to remember that neither manual nor
collared stabilization provides true immobilization.
The jaw thrust maneuver should be
attempted in these cases by lifting the jaw forward
with the provider’s third or fourth fingers (or both) “hooked” under the angles
of the mandible while avoiding compression of the soft tissues. The goal is to
pull the mandibular block of tissue forward so that the lower central incisors
are anterior to the upper central incisors.
At any point, if it is determined
that the patient is unable to independently maintain the patency of his or her
airway, the provider should open the airway to maintain adequate ventilation
and protection from the aspiration of stomach contents. Simple suctioning
should be attempted first because it may relieve an airway obstructed by
secretions or foreign materials.
 |
| Figure 1-2 Head tilt–chin lift and jaw thrust maneuvers. Figure 1-3 Oral and nasopharyngeal airways. |
Bag–valve–mask (BVM) ventilation
(see below) may provide an open airway if a good mask seal is achieved and
airway positioning is maintained.
In an unconscious patient, an
oropharyngeal airway can be used to help stent the mandibular block of tissue
away from the posterior hypopharynx (Figure 1-3). A nasopharyngeal (NP) airway
is another option. NP airways are well tolerated in unconscious and
semiconscious patients and may even be used in conscious individuals with upper
airway obstruction. NP airways should be used with caution when midface trauma
is suspected because of the risk of inserting the airway through fractured bone
into intracranial structures. Laryngeal mask airways (LMAs) are supraglottic
airway devices that are being increasingly used in resuscitation settings to
help bypass the soft tissues of the anterior oropharynx and to deliver oxygen
directly to the proximal trachea.
When the patency of the airway
cannot be maintained by other means, endotracheal intubation offers a
relatively stable artificial airway. The following formula is used to determine
the appropriate size (inner diameter) for an uncuffed endotracheal tube (ETT):
(Age in years/4) + 4. Cuffed ETTs are being used with increasing frequency in
children. The appropriate size can be determined using the above formula and
then subtracting 0.5 (mm) from the result. One
should have tubes one size larger and smaller at the ready in case this
estimate is not quite appropriate for the individual
patient. An appropriately sized laryngoscope blade should be chosen; the two
most common types are Miller (straight) and Macintosh (curved) blades.
Pharmacologic agents (premedications
such as atropine and lidocaine, sedatives, and paralytics) may be used to
increase patient comfort, improve patient safety, and increase the chances of
successful intubation. These are not necessary when the patient presents in
cardiopulmonary arrest. In other settings, premedications should be selected
based on the clinical scenario and prepared to be given. All equipment should
be checked before sedative or neuromuscular blocking agents are administered to
the patient. A useful mnemonic for preparing materials for intubation is “SOAP
ME” for suction, oxygen, airway equipment, pharmacy or personnel, and
monitoring equipment. After medications have been given, it is important to
preoxygenate the patient using 100% oxygen via facemask.
Direct laryngoscopy can be
accomplished by positioning the patient’s head and then using the right thumb
and index finger to “scissor” open the mouth. The laryngoscope blade is
inserted under direct vision toward the right corner of the mouth over the
tongue and over the epiglottis (if using a straight blade) or into the
vallecula (if using a curved blade). The tongue should be “swept” toward the
left side the mouth while the laryngoscope handle is pulled upward at a
45-degree angle, taking care not to damage the teeth or gums (Figure 1-4).
Suctioning may be needed to clear secretions
to visualize the vocal cords, which should fall into the direct line of sight.
The provider should maintain his or her view of the larynx and insert the ETT
while watching it pass through the vocal cords. The tube should be placed so
that the second of the distal vocal cord markers is at the level of the vocal
cords. A projection for how deep to place the tube (centimeter mark at the
teeth) can be calculated using the following formulas: [(Age in years/2) + 12]
or [3 × (External diameter of the ETT)].
 |
Figure 1-4 Endotracheal intubation.
Confirmation of proper insertion of
the ETT can be accomplished in several ways. Primary confirmation should always
be confirmed by the detection of exhaled carbon dioxide (CO2)
through the use of a colorimetric CO2 detector or use of inline
capnography. Capnography has the added advantage of showing exhaled levels of
CO2. In patients in cardiopulmonary arrest, exhaled CO2
may not be present even with proper tube placement. Listening for symmetric
breath sounds in bilateral lung fields, observing symmetric chest wall rise,
and maintaining a good oxygen saturation are all secondary signs of good tube
placement. Visualizing mist in the ETT with expiration is helpful but may occur
with misplacement of the tube into the esophagus as well. When time permits, a
chest radiograph should be obtained to confirm placement, including depth of
the ETT. The tip of the tube should be approximately 1 cm above the carina.
Breathing
After the airway has been
stabilized, assessment of ventilation and gas exchange should be initiated.
Observation of chest wall movement can provide clues regarding adequacy of
respiratory effort. In infants, adequate chest wall movement is characterized
by uniform expansion of the lower chest and upper abdomen. In older children
and adolescents, observation should focus on upper chest expansion.
Auscultation over the trachea assesses central airway patency. Breath sounds should
then be auscultated over the upper lung fields while focusing on adequacy of
air movement and symmetry of breath sounds. Adequate gas exchange can be
assessed using pulse oximetry, capnography, and blood gases. Because hypoxia is
the major means of pediatric cardiac arrest, supplemental oxygen should be
given to all critically ill patients to maximize oxygen delivery.
 |
Figure 1-5 Maintaining airway patency and securing the mask in bag–valve–mask ventilation.
If the patient’s efforts at ventilation or oxygenation are compromised, assisted ventilation should be initiated. BVM ventilation (Figure 1-5) is a skill at which all physicians working in acute-care settings should become adept. Masks of various sizes should be available, and the smallest mask that completely covers the mouth and nose should be selected. Airway patency is maximized when the patient’s head is placed in the “sniffing position,” with the neck slightly flexed while the head is rotated into extension.
When using the chin lift maneuver,
the provider’s nondominant hand should be used to hold the mask in place by
forming a “C” around the connector with the thumb and index fingers while the
remaining fingers maintain the chin lift along the angle of the mandible. If
the jaw thrust is used, the mask should be secured with the thumb and index
fingers of both hands with the remaining third or fourth fingers maintaining
the jaw thrust at the angles of the mandible. Downward pressure on the mask
should be used to provide countertraction against the upward force generated by
the jaw maneuver, maintaining an adequate seal of the mask against the face.
The provider should concentrate on trying to “lift” the jaw up to the mask.
With either maneuver, the mask should fit snugly on the face, and the provider
should assess for an adequate seal (attempting to minimize air leaks), which is
the most important aspect of effective BVM ventilation.
After an adequate seal has been
achieved, ventilation can be accomplished
by administering positive pressure via the resus- citation bag. A two-person
technique is preferred, with one person holding the mask in place and the other
providing breaths. The amount of positive pressure generated should be dictated
by the adequacy of chest wall excursion—the patient’s chest wall movements
should be similar to normal deep respirations. The recommended number of respirations
depends on age—infants and children should receive 15 to 20
breaths/min (≈1
breath every 3-5 seconds), and adolescents should receive 10 to 12 breaths/min
(≈1 breath every 5-6
seconds).
Although BVM ventilation is a safe
procedure, there are potential complications.
Equipment failure is a common and avoidable complication that may lead to
inadequate oxygenation and ventilation. Oxygen sources and the patency of
connections should be checked routinely as well as when problems arise. Other
complications of BVM ventilation include cervical cord damage in cases of
traumatic injury, hyperventilation, pneumonitis
associated with reflux and aspiration of stomach contents, pneumothorax, and
gastrointestinal tract distension.
Circulation
The goals of the circulatory
assessment are to evaluate cardio- vascular function and end-organ perfusion.
Cardiovascular dysfunction can be reflected by changes in skin color,
temperature, heart rate, heart rhythm, blood pressure, pulses, and capillary
refill time. End-organ dysfunction can be reflected by changes in brain
perfusion (manifesting as altered mental status), skin perfusion, and renal
perfusion (manifesting as decreased urine output).
Heart rate should be appropriate for
the child’s age (Table 1-2) but may be affected by clinical conditions other
than poor circulation (e.g., fever, dehydration, pain). Normal blood pressure is also age dependent (Table 1-3) and
can also be affected by associated clinical conditions. In children,
compensatory mechanisms (tachycardia, increased stroke volume, and
vasoconstriction) may cause blood pressure to be preserved even though there is
inadequate tissue perfusion. This is termed compensated shock (see
Chapter 2). However, hypotension should be treated as shock until proven otherwise
in critically ill or injured children because it represents a state in which
compensatory mechanisms have failed (uncompensated shock). It is important to
measure blood pressure using a properly sized cuff.
Assessment of perfusion should
include palpation of both central (most commonly femoral) and peripheral
(radial and dorsalis pedis) pulses. In infants, the brachial pulse is checked.
Weak central pulses portend impending circulatory failure. A discrepancy
between central and peripheral pulses may suggest worsening shock but may be
caused by appropriate vasoconstriction in a cold environment. Prolonged
capillary refill times may also be seen during times of inadequate perfusion.
However, both ambient temperature and the patient’s body temperature may cause
prolonged capillary refill to be a nonspecific finding. Compromised circulation
may be the result of a number of different factors, including blood loss,
dehydration, neurologic injury, and infection. Ideally, during the primary
assessment, providers obtain vascular access. Large-bore intravenous (IV) lines
are preferable; however, it may be difficult to establish venous access in a
critically ill child who has compromised per-fusion. The intraosseous (IO)
route is a quick and reliable technique. Newer IO devices have been developed
(including spring-loaded needles and battery-powered handheld drills), which may be easier to use, especially in larger
children. Fluid resuscitation is indicated in states of circulatory compromise.
The goal is to prevent cardiopulmonary arrest, which is the cessation of blood
circulation resulting from ineffective or absent cardiac activity.
 |
Figure 1-6 Arrest rhythms.
Cardiac arrest is associated with the following arrest rhythms (Figure 1-6): asystole, PEA, VF, and pulseless VT. Asystole is characterized by the absence of discernible electrical activity (“flatline”). PEA is a condition in which the patient has no palpable pulse despite showing electrical activity on cardiac monitoring (but excludes VF, VT, and asystole). VT is characterized by organized, wide QRS (>0.08 sec) complexes. Pulseless VT must be distinguished from VT with a pulse because they are treated differently. VF is a form of pulseless arrest that is characterized by chaotic, disorganized electrical activity on cardiac monitoring with an absence of coordinated contractions. For all of these rhythms, it is important to provide supplemental oxygen (100%) and to initiate CPR immediately.
CPR (Figure 1-7) is indicated for
the management of cardiopulmonary arrest. Studies have consistently shown that
CPR, when performed correctly, saves lives. The mantra “push hard, push fast,
minimize interruptions, allow full chest recoil, and do not overventilate”
should guide the provider’s efforts.
Recommendations for compression to
breath ratios are summarized. In newborns, a ratio of 3:1 is recommended. In
infants and children, the compression to ventilation ratio is different
depending on whether there is a single rescuer or two rescuers performing CPR.
A single rescuer should give 30 compressions for every 2 breaths, whereas 2
rescuers should perform CPR using the ratio of 15 compressions for every 2
breaths. In both scenarios, the goal is to provide at least 100 compressions
per minute. After an artificial airway has been placed, continuous compressions at a rate of at least 100
compressions/min with ventilations at a rate of about 8 to 10 breaths/min (1
breath every 6-8 sec) should be performed. Resuscitation of the newly born
infant is discussed in Chapter 106.
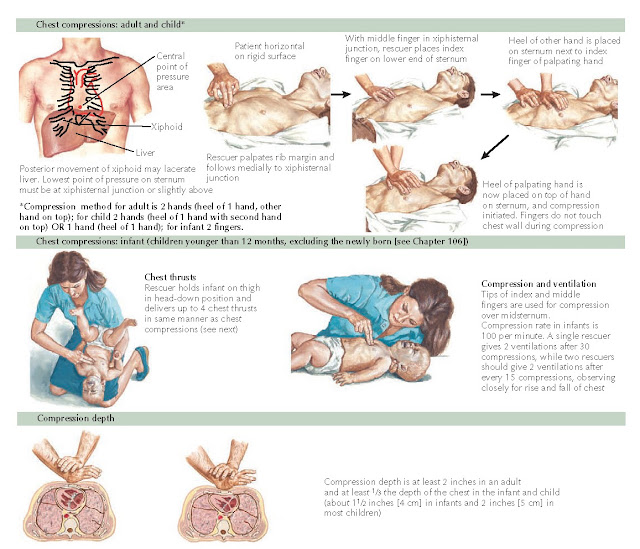 |
Figure 1-7 External chest compressions.
Synchronized cardioversion is indicated for VT accompanied by a pulse. The initial dose is 0.5 to 1 J/kg. If ineffective, an additional dose of 2 J/kg should be administered. Defibrillation is indicated for VT without a pulse and VF. If using a manual defibrillator, the initial dose is 2 J/kg, and an automated external defibrillator (AED) may be used for patients older than 1 year of age.
Although a full discussion of
resuscitation medications is beyond the scope of this chapter, it is important
to be familiar with the most important pharmacologic agents used during resuscitation. Epinephrine, an adrenergic agent
with both α-
and β-agonist
properties, is used to promote vasoconstriction, which is important in
increasing aortic diastolic pressure and coronary perfusion pressure. It also
increases automaticity of cardiac muscle and makes cardiac muscle more
susceptible to the effects of cardioversion and defibrillation. It is used as a
first-line agent in pulseless arrest with nonshockable rhythms (i.e., asystole
and PEA) and symptomatic bradycardia (heart rate <60 beats/min with poor
perfusion despite CPR). It is also used for VF and pulseless VT that do not
respond to defibrillation. Although the dose and concentration used are route
dependent (0.01 mg/kg of 1:10000 IV or IO and 0.1mg/kg of 1:1000 via ETT), the
appropriate volume to give is easily remembered as 0.1 mL/kg. CPR should be
continued for at least one full cycle (2 minutes) of compressions after any
intervention and until medications for the treatment of cardiorespiratory
arrest have taken effect.
After the return of spontaneous
circulation, it is important that meticulous attention be paid to
post-resuscitation care including glucose monitoring and control, fluid
resuscitation, and support of cardiovascular stability through vasoactive
medications.
Disability
Disability assessment focuses on
evaluation of the two main components of the central nervous system, the
cerebral cortex and the brainstem. A number of scales are used to assess neuro-
logic function. The AVPU Pediatric Response Scale is quick and simple to apply
in critical situations. Level of consciousness is described as:
A: alert (child is awake, active,
and appropriately responsive) V: voice (child responds to voice)
P: pain (child responds only to
painful stimulus)
U: unresponsive (child does not
respond to any stimulus)
A more detailed assessment for older
children and adolescents, the Glasgow Coma Scale, is the most widely used
method (Figure 1-8). The patient’s best responses in each of the
categories (eye opening, verbal response, motor response) are added to produce
a score out of 15. A change of 2 points reflects a clinically significant
change in neurologic status. This scale is modified
when used in infants and younger children.
The pupils should also be examined
during this part of the assessment to help assess brainstem function. Normally,
the pupils constrict in response to light and dilate in dark environments. An
abnormal size of pupils, failure of pupils to react to light, and asymmetry of
pupil size are all abnormalities that should be noted during the primary
assessment.
An additional “D” that is included
for a noninjured, critical patient is a “D-stick” (fingerstick glucose
measurement) because many medical conditions leading to critical illness are
characterized or accompanied by disturbances in blood sugar. For the treatment
of hypoglycemia, the appropriate dose of dextrose to administer as an IV bolus
is 0.5 to 1 g/kg, which is equivalent to 5 to 10 mL/kg of D10W (dextrose 10% in
water).
Exposure
Complete exposure by undressing an
injured or ill child should ideally be accomplished simultaneously by ancillary
staff while the other components of the primary survey are addressed. Complete
exposure is important to facilitate a comprehensive physical examination as
part of the secondary survey. Another important “E” is “environment.” The
provider should take care to institute warming measures for the exposed child
if clinically indicated, and the environment should be free of contaminants
that may exacerbate the child’s clinical condition (e.g., a child who experienced inhalation
injury should be taken out of the smoky environment).
SECONDARY ASSESSMENT
After completion of the primary
assessment, including addressing any abnormalities discovered during the course
of evaluation, the provider should initiate the secondary survey, which
includes a focused history and physical examination. The SAMPLE mnemonic is
helpful in addressing the important parts of the focused history: signs
and symptoms, allergies, medications,
past medical history, last meal, and events
leading to current condition. Questions should be directed toward
attempting to determine factors that may help to explain impaired respiratory,
circulatory, or neurologic function. A focused physical examination is best
approached in a head-to-toe fashion.
FUTURE DIRECTIONS
Outcomes from pediatric
resuscitation have improved incrementally over the past several decades.
Research has brought about advances in our understanding of the pathophysiology
and management of cardiopulmonary arrest and its consequences. Current areas of
research include therapeutic hypothermia, oxygen toxicity and reperfusion
injury, the molecular genetics behind causes of cardiopulmonary arrest, and
genetic polymorphisms and their implications in response to therapy. Pre- and
postconditioning of the myocardium and brain epithelium, emergency preservation
and resuscitation (EPR), postresuscitation myocardial support, mechanical
circulatory support, quality CPR, and the epidemiology of CPR are also subjects
of significant inquiry. The once dismal prognosis of critically ill and injured children continues to
improve as discoveries of promising therapeutic
advances are made in pre- and postresuscitation
care.






