RENAL ABLATION
Nephron-sparing surgery has become the standard of care for patients with small (<4.0 cm) renal masses (SRM). For young, healthy patients with a low surgical risk, open or laparoscopic partial nephrectomy (OPN/LPN) is preferred. Renal tumor ablative techniques, however, are relatively new developments with increasing application. Such techniques were initially indicated in patients with multiple renal tumors, a solitary kidney, or significant comorbidities that precluded higher risk surgery. In contemporary practice, however, sufficient evidence indicates that ablation may be a reasonable treatment option for all patients with SRMs. The advantages of renal ablation over LPN include less blood loss, shorter hospitalization time, decreased post-operative pain, and a lower complication rate.
 |
| Plate 10-24 LAPAROSCOPIC CRYOABLATION: RETROPERITONEAL APPROACH |
ABLATION
MODALITIES
At present, the clinically viable ablation technologies include cryoablation and radiofrequency ablation. Either can be performed using laparoscopic or percutaneous technique. Both involve placement of probe needles directly into the renal mass.
In
cryoablation, the cryoprobe needles are cooled to very low temperatures, which
induces tissue necrosis. At present, such cooling is achieved by delivering
pressurized argon gas to the tips of the cryoprobes. As argon gas passes
through the restricted tips of the probes and then expands, it undergoes rapid
cooling (a phenome-non known as the Joule-Thomson effect) and forms an iceball
over the tumor. The temperature at the probe tip becomes as low as -140° to
-190° C, whereas the temperature at the edge of the ice ball is just below 0°
C. Since the temperature required for cell destruction is between -20° and -40°
C, the efficacy of the ablation process declines in a gradient radiating from
the tips of the probes toward the edges of the iceball. Therefore, the iceball
must involve a margin of normal tissue to ensure complete tumor destruction.
Following the freeze cycle, an active thaw phase is initiated, and then a
second freeze-thaw cycle is performed to further increase cell death.
In
radiofrequency ablation (RFA), the probes transfer high-frequency electrical
current to the target tissue, which results in the production of thermal
energy. Temperatures in excess of 60° C cause tissue destruction through
coagulative necrosis and thermally induced vascular thrombosis.
High-intensity
focused ultrasound (HIFU) is an experimental extracorporeal procedure in which
focused ultrasound waves pass through the skin and are converted to heat
energy at a selected target.
Contemporary
HIFU technologies, however, have not demonstrated adequate oncologic efficacy
and are thus not yet part of standard clinical practice.
TECHNIQUE
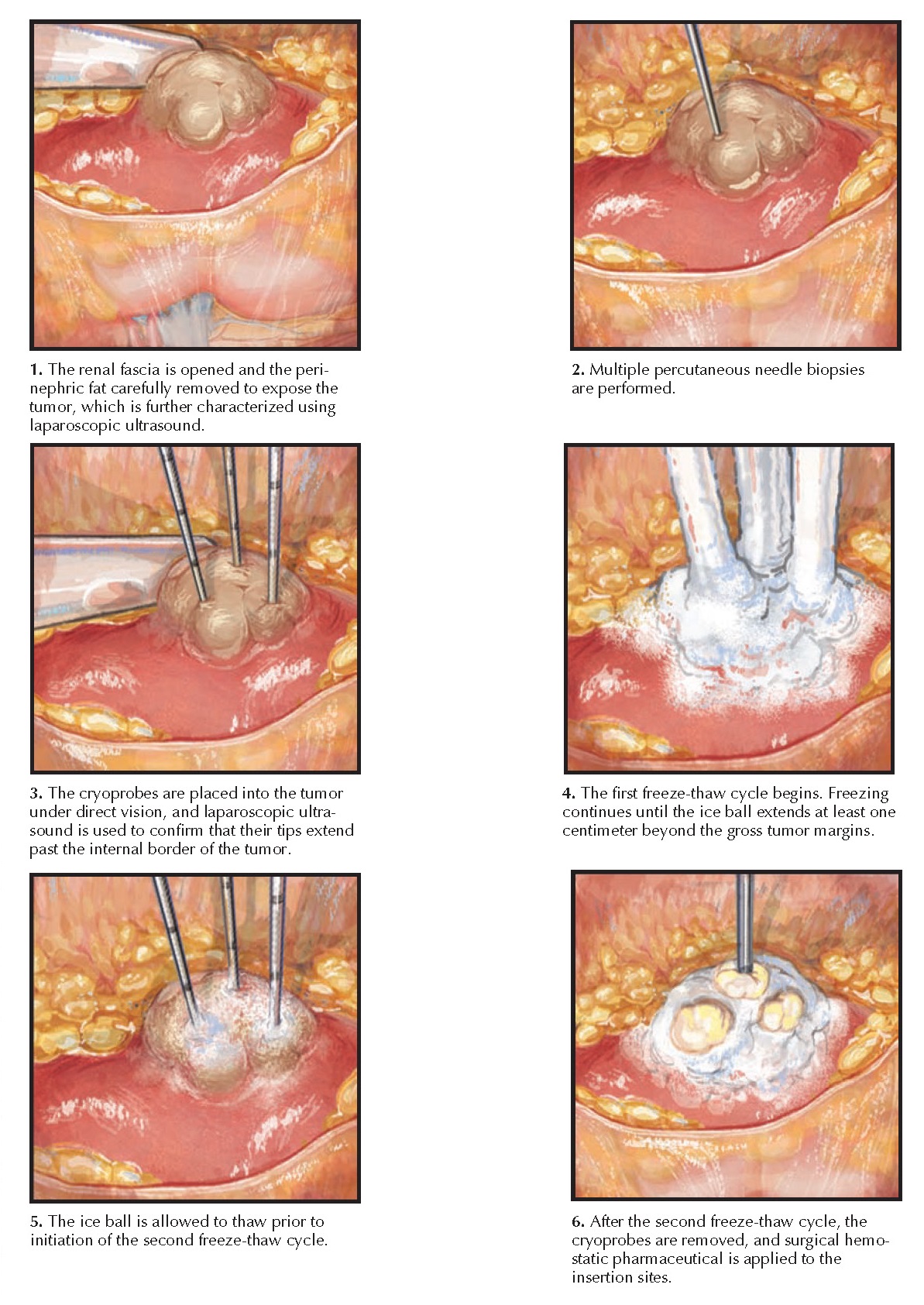
Laparoscopic
Technique. In a laparoscopic ablation, the tumor can be directly visualized, and
the ablation process can be monitored in real time.
The tumor
can be accessed from either a transperitoneal or retroperitoneal approach,
depending on its location. In the transperitoneal approach, the colon is mobilized
medially to expose the renal fascia, which is mobilized from its attachments to
surrounding structures, such as the liver or spleen. Next, the renal fascia is
entered over the area of the renal mass, which is targeted using preoperative
imaging and intraoperative ultra-sound. The perirenal fat is then carefully
removed from the renal capsule to
expose the entire surface of the tumor.
Once the
tumor has been adequately visualized, multiple core biopsies are acquired using
a percutaneous biopsy device. Intraoperative ultrasound is then performed to
further characterize the tumor’s depth and vascularity.
Finally,
ablation probes are inserted through the skin and into the tumor under direct
vision. The number of ablation probes that should be deployed depends both on
the tumor’s characteristics and the technology being applied. The probes should
enter the tumor at a right angle, and laparoscopic ultrasound should be
performed to ensure the probe tips are beyond the internal margin of the tumor.
Once proper positioning has been achieved, the probes are activated.
During
cryoablation, laparoscopic ultrasound can be used to monitor the iceball as it
forms, ensuring that it completely engulfs the mass and a 1-cm rim of normal
parenchyma. Two freeze-thaw cycles are performed, and then the probes are
removed. To minimize the chance of bleeding, probe extraction should not be
attempted until the probes are loose enough to freely twist within the tumor.
During RFA,
the ablation process cannot be visualized in real time. Instead, the RF
ablation process proceeds using temperature- or impedance-based algorithms that
are device-specific. Alternatively, some clinicians deploy temperature probes at
selected sites around the tumor to monitor the ablation process.
After the
ablation process is completed, the lesion is monitored for hemorrhage, and
minor bleeding is controlled using topical hemostatic agents and gentle
pressure. After hemostasis has been confirmed at reduced pneumoperitoneum, the
trocars are removed.
Percutaneous
Technique. Percutaneous ablation offers numerous advantages over a laparoscopic
procedure, including avoidance of general anesthesia, reduced complication
rate, diminished postoperative pain, and expedited convalescence. The major
disadvantages, however, include the lack of direct visualization during the
ablation process, as well as the inability to assess for immediate postablation
bleeding.
Percutaneous
ablation may be performed in a CT or magnetic resonance imaging (MRI) suite.
The patient is placed under conscious sedation and positioned prone. A
semipermeable targeting template is positioned over the ipsilateral flank, and
imaging is performed to correlate the template with the renal anatomy. The
targeting template is marked to indicate the location where the needles should
be inserted, and then the template is removed so that the mark is visible on
the patient’s skin. The site is sterilized and draped in standard fashion. An
access sheath is then deployed at the
marked site,
and its position in the kidney is confirmed and readjusted if necessary using imaging.
Several tumor biopsies are acquired through the access sheath. The first probe
is then placed through the access sheath. Subsequent probes are placed directly
through the skin, with additional imaging performed to confirm proper
positioning. The ablation is then performed. Once completed, a final image
series is acquired using a half dose of intravenous contrast to confirm
successful tumor ablation.
 |
| Plate 10-25 PERCUTANEOUS CRYOABLATION |
COMPLICATIONS
Both
cryoablation and RFA tend to have lower complication rates than OPN/LPN.
Nonetheless, they carry a risk of some major complications, including bleeding
from the tumor, injured intraabdominal vessels, or skin; pain at the trocar or
probe sites; urinary tract infection; intraabdominal abscess; ileus; injury to
adjacent organs; and tumor persistence or recurrence after treatment.




