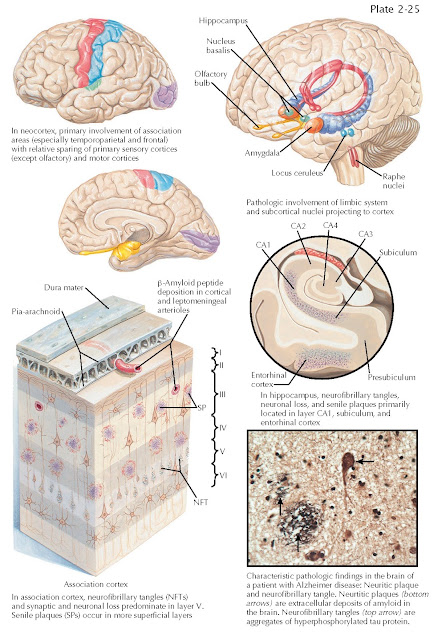
ALZHEIMER DISEASE: DISTRIBUTION OF PATHOLOGY
The pathologic diagnosis of Alzheimer disease is determined at autopsy based on the presence of its cardinal histopathologic features, neurofibrillary tangles, and amyloid plaques.
Amyloid plaques are abundant in the
cerebral cortex of individuals with Alzheimer disease, particularly in the
parietal and frontal regions. Amyloid deposition is also commonly observed in
leptomeningeal arteries as amyloid angiopathy. Autopsy studies, and more
recently amyloid imaging techniques, have revealed that amyloid plaques begin
to accumulate in the brain years, perhaps decades, before the emergence of
clinically recognizable symptoms and are found in cortical regions that are
highly metabolically active, such as the default-mode network that is active
when an individual is at rest and not engaged in a specific cognitive task.
Regions such as the precuneus and posterior cingulate, which have strong
connections with the hippocampus, are among the areas affected earliest.
Neurofibrillary tangles (NFTs)
accumulate in a predictable fashion as an individual ages and is a ubiquitous
accompaniment of aging. Accumulation of neurofibrillary tangles begins in the
medial temporal lobe (amygdala and entorhinal cortex) gradually extending into
the limbic system (hippocampus and cingulate cortices) and later throughout the
entire isocortex. This stereotypic pattern of accumulation is used in
pathologic staging of the disease (Braak staging). The pathologic staging of AD
is based on the hierarchic pattern of the appearance of neurofibrillary tangles
in various regions. There are two “presymptomatic” transentorhinal stages,
where NFTs remain in the perirhinal cortex. In stage III, the NFTs involve the
limbic regions, and layer II of the entorhinal cortex. Stage IV AD is marked by
more extensive NFTs in the limbic regions, entorhinal layer IV, and hippocampal
CA1 region. These latter stages (III and IV) correspond clinically to mild
cognitive impairment (MCI), not dementia. MCI represents an intermediate stage
between normal aging and dementia. Typically, patients note subjective memory
problems, the need to make lists, and short-term memory “slip ups,” but these
changes are not severe enough to interfere with day-to- day activities. As the
pathologic stage of AD progresses, the NFTs accumulate in the inferotemporal,
retrosplenial, and, eventually, association regions of the cortex, while the
primary motor cortex is spared. In these stages, the clinical hallmarks of AD
are present and include impairments in memory, judgment, orientation, language,
and decision-making.
Of interest, some tangle pathology
is present in all older adults, although individuals with AD have a greater
burden of neurofibrillary tangles and a much more widespread distribution
throughout the isocortex. The CA1 region of the hippocampus and the entorhinal
cortex are particularly susceptible to the accumulation of both plaques and
tangles in the early stages of the disease. These regions are important for
mediating the formation of memories, and their degeneration
accounts for the prominent impairments in short-term memory observed in AD
patients.
Biochemical data from patients with
AD reveal an early decrease in choline acetyltransferase and acetylcholinesterase,
indicating dysfunction in the neural pathways that use acetylcholine as a
neurotransmitter. The number of neurons is reduced in the basal nucleus of
Meynert, which has widespread
cholinergic neuron innervations through most of the cerebral cortex. Selective
degeneration of the basal forebrain cholinergic neurons results in a
cholinergic deficit that contributes to AD symptoms. These findings led to the
development of the first effective treatments in ameliorating the symptoms of
AD, acetylcholinesterase inhibitors, which act by increasing acetylcholine
levels in the brain.