RENAL BIOPSY
A renal biopsy yields a small piece of renal parenchyma for histopathologic examination. Because many renal diseases have essentially indistinguishable clinical findings, renal biopsy is often crucial for establishing the correct diagnosis and devising an effective treatment plan. The procedure is generally uncomplicated and, in most cases, can safely be performed by a nephrologist at the bedside.
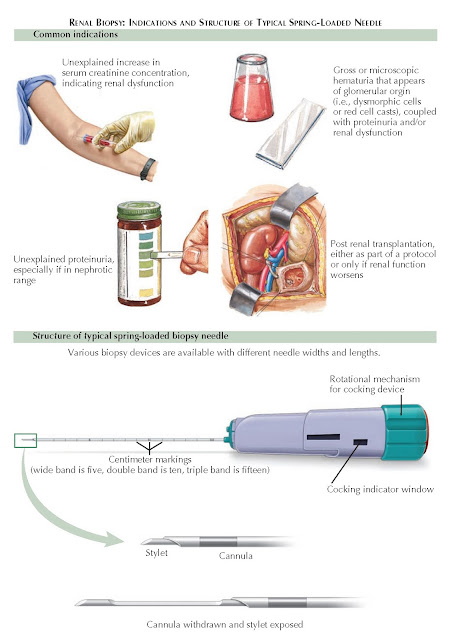
Plate 10-7
RENAL BIOPSY: INDICATIONS AND STRUCTURE OF TYPICAL SPRING-LOADED NEEDLE
INDICATIONS
The major
indications for renal biopsy include renal failure of unknown cause,
proteinuria, hematuria, and renal transplantation.
Proteinuria.
In
patients with mild proteinuria (1 to 2 g/day) that has no obvious cause, such
as diabetes mellitus, a renal biopsy may be performed to establish a definitive
diagnosis. The exact threshold for biopsy differs across practitioners and
depends on individual clinical judgment. Possible causes of this degree of
proteinuria include glomerulonephritis and mild forms of the diseases that
typically cause nephrotic syndrome, such as focal segmental glomerulosclerosis
(FSGS) or membranous nephropathy (MN). Although tubulointerstitial disease
commonly causes mild proteinuria, a biopsy is generally not required to
establish the diagnosis.
In patients
with nephrotic-range proteinuria (i.e.,3 g/day), a renal biopsy is indicated
to identify the disease process, guide treatment, and determine prognosis.
Possible causes of nephrotic-range proteinuria include primary or secondary
FSGS, MN, minimal change disease (MCD), and (rarely) fibrillary or immunotactoid
glomerulonephritis. If, however, the patient has a diagnosed systemic illness
that is known to cause nephrotic syndrome, a renal biopsy is typically not
required. Examples include patients with long-standing diabetes mellitus and
concurrent diabetic retinopathy, or patients with amyloidosis seen on a biopsy
of another affected organ system. In addition, young children with nephrotic
syndrome are generally presumed to have MCD, with a renal biopsy only performed
if empiric treatment for this condition fails.
Hematuria.
In
patients with gross or microscopic hematuria, the initial workup should focus
on urologic abnormalities, such as nephrolithiasis, neoplasm, or infection. The
presence of dysmorphic red cells, proteinuria, and renal insufficiency, however,
strongly points toward glomerular disease. Many renal diseases are associated
with microscopic hematuria, including essential hematuria, acute interstitial
nephritis, IgA nephropathy, membranoproliferative glomerulonephritis,
postinfectious glomerulonephritis, lupus nephritis, cryoglobulinemia,
fibrillary/immunotactoid glomerulonephritis, ANCA-associated vasculitis,
malignant hypertension, atheroembolic renal disease, renal infarction,
thrombotic microangiopathy, Henoch-Schönlein purpura, thin basement membrane
nephropathy, hereditary nephritis, and anti-GBM disease. A kidney biopsy is
essential for establishing the correct diagnosis and determining an optimal
treatment plan.
Occasionally,
patients may have isolated hematuria (i.e., without proteinuria or renal
insufficiency). The differential diagnosis for such patients includes thin basement membrane
disease, mild IgA nephropathy, and hereditary nephritis. A kidney biopsy is
typically not performed, however, because treatment is not instituted unless
there is significant proteinuria or renal insufficiency.
Renal
transplant. Patients who have undergone renal transplant and subsequently develop
renal failure should have a biopsy if their renal function does not improve
after provision of intravenous fluids. In such circumstances, a biopsy is
helpful for differentiating between various entities, such as acute or chronic
rejection, drug toxicity (especially from calcineurin
inhibitors), and BK virus infection. Some centers also routinely take biopsies
from transplanted kidneys at predetermined time points, even in the absence of
overt dysfunction because some renal disease may initially be clinically
silent.
PROCEDURE
Before a
patient undergoes a renal biopsy, anticoagulation medications should be
stopped, and bleeding risk should be evaluated by obtaining a prothrombin time,
partial thromboplastin time, and platelet count. Any bleeding diathesis should
be corrected, if possible, before the procedure.
Most
patients can undergo a percutaneous biopsy, which is performed at the bedside;
however, select patients may require alternate approaches, including open,
laparoscopic, and transjugular biopsies. The major indications for these
techniques include an uncorrectable bleeding diathesis, morbid obesity,
solitary kidney, infection of the skin over the kidneys, and failed
percutaneous attempts.
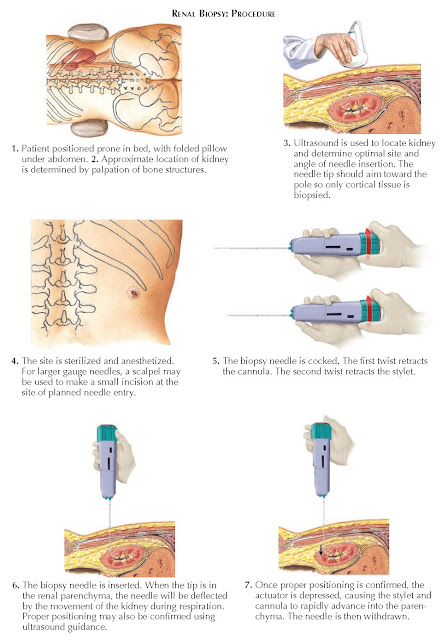
For a
percutaneous biopsy, most patients should be placed prone, with a folded pillow
under the abdomen. An ultrasound is performed to visualize the kidney and
determine the location and angle of needle insertion. The upper or lower pole
should be targeted so that only cortical tissue is acquired. Hydronephrosis,
multiple cysts, or small hyperechoic kidneys may be seen, which increase the
bleeding risk and should be considered relative contraindications.
Once the
initial ultrasound is complete, the site is dressed and draped in normal
sterile fashion. The site is injected with a local anesthetic, and a scalpel
may be used to nick the skin at the area of planned needle insertion. The
biopsy needle, which consists of a spring- loaded outer cannula and inner
stylet, is then cocked as shown in the diagram. The needle is passed through
the skin into the renal parenchyma, often using ultrasound for real-time
guidance. The patient is instructed to hold his or her breath, and then the
actuator button is depressed, causing rapid advancement of both the inner
stylet and outer cannula to the device’s predetermined penetration depth. A
tissue core is acquired as the cannula rapidly passes over the stylet. Two or
three cores should be acquired to ensure an adequate sample. The adequacy of
the tissue cores can be assessed using low-power microscopic examination. An
adequate sample should contain a minimum of 8 to 10 glomeruli. The cores should
be transported in normal saline to the pathology laboratory or placed in
fixatives if the laboratory is not on site. A renal pathologist then examines
the tissue using light microscopy, electron microscopy, and immunofluorescence
or immunohistochemistry. Typical
routine stains for light microscopy include hematoxylin and eosin, periodic acid–Schiff, Jones’ silver methenamine, and
trichrome.
COMPLICATIONS
The main
complications of a renal biopsy include bleeding, pain, damage/puncture of
surrounding structures (liver, spleen, bowel), and arteriovenous fistula
formation. Bleeding is by far the most common complication, and it can occur
into the urine collecting system,
perinephric space, or subcapsular space. Patients should thus be monitored for
approximately 4 to 6 hours after the procedure, with vital signs, hemoglobin
levels, and urine color noted. Some centers perform a follow-up computed
tomography (CT) scan or ultra-sound a few hours after the biopsy. In the event
of a major bleed, transfusions or therapeutic procedures (e.g.,
angioembolization or laparotomy) should be performed as needed. In very rare
cases, a renal biopsy results
in kidney loss or death.