MANAGEMENT OF LIPID ABNORMALITIES
The management of lipid disorders in reducing the risk of coronary heart disease (CHD) has evolved in the past few years. There are a number of factors that account for these changes the introduction of the 2013 American Heart Association/American College of Cardiology (AHA/ACC) guideline report on cholesterol management and a series of clinical trials on nonstatin therapies (notably, several trials involved the cholesteryl ester transfer protein inhibitors [CETPis] for high density lipoprotein [HDL] elevation), as well as the introduction of proprotein convertase subtilisin/kexin type 9 (PCSK-9) therapies. The aforementioned 2013 recommendations are a key resource because of their evidence-based approach to patient care. They have simplified both the treatment approach to lipids and challenging issues such as dose titration, as well as achieving a specific and perhaps unreachable “target” lipid value. Of great importance, they allow for discretion on the part of the provider to engage with the patient in shared decision making and as stated, “Guidelines attempt to define practices that meet the needs of patients in most circumstances and are not a replacement for clinical judgment.”
For most individuals at risk for CHD, elevated serum lipid levels
specifically, elevated low-density lipoprotein cholesterol (LDL-C) are the dominant modifiable risk factor.
The importance of lifestyle modification, inclusive of diet and exercise,
cannot be understated in the coordinated effort to reduce vascular disease
risk. A case example of individuals at high vascular risk are those determined
to have the metabolic syndrome (Fig. 16.1). Together with appropriate medical
management, therapeutic lifestyle modification represents an important and
effective approach to overall patient management.
LDL-C levels are strongly associated with atherosclerosis and CHD events.
Insights from genetic, epidemiological, and multiple clinical trial data
reinforce the belief that LDL-C is a necessary and sufficient cause of
atherosclerosis, and therefore, most emphasis is placed on lowering LDL-C.
There appears to be a consistent graded reduction in risk in CHD events
associated with lowering of LDL-C levels with drug and diet therapy. The 2013
AHA/ACC guidelines summarize the evidence base for lowering LDL-C with statins
within four distinct patient groups based on their future risk for
cardiovascular events. These groups are described as follows (Fig. 16.2):
· individuals with known clinical atherosclerotic
cardiovascular disease (ASCVD);
· individuals with primary elevations of LDL-C to
>190 mg/dL, typically seen in genetic dyslipidemia;
· individuals with diabetes, aged 40 to 75 years,
with LDL-C of 70 to 189 mg/dL without
clinical ASCVD; and
· patients without clinical ASCVD or diabetes with
LDL-C of 70 to 189 mg/dL and an
estimated 10-year ASCVD risk of >7.5%.
These statin guidelines are fundamental to lipid management, and
additional guidance on nonstatin therapies are now available through a 2017
Focused Update from the ACC Expert Consensus Decision Pathway. This update provides expert guidance on individuals who respond inadequately to statin therapy or
may not be able to tolerate maximum doses of statins. Drugs such as ezetimibe
and the PCSK-9 inhibitors offer an important option of additional lowering of
LDL-C and reducing cardiovascular risk. Alternative therapies, which include
likely referral to a lipid specialist, other agents such as mipomersen or
lomitapide, or LDL apheresis may also be considered for selected patients.
ASSESSMENT
Standard laboratory lipids measured by β-quantification consist of total
cholesterol, triglycerides (TGs), and HDL-C levels as direct measurements, and
LDL-C as estimated from the Freidewald equation. Direct measurement of LDL-C
levels, particle size, and particle density are performed by
ultracentrifugation, gradient gel electrophoresis, and nuclear magnetic
resonance. Although measurement of apolipoprotein B and these other measures of
LDL-C may provide additional information on lipid lipoprotein characteristics,
detailed clinical studies that indicate the usefulness of drugs that target
these individual lipid com- ponents are not available. For this reason, the
usefulness of these measures may be of limited value because they rarely change
management decisions for most patients. LDL-C measurement is the standard for
evaluating risk and monitoring lipid therapy. For patients being considered for
long-term therapy, two fasting measurements of the lipoprotein profile, taken
at least 1 week apart, should be obtained to support a clinical decision.
The fasting TGs are also important to monitor, because elevated TGs
(>200 mg/dL) may mask residual risk in the form of very low-density
lipoprotein and other remnant cholesterol particles, which are also considered
atherogenic.
The goal of therapy then is to match the intensity of LDL-C lowering with
individual patient risk; for example, an individual with known ASCVD would be
managed with a high-intensity statin that provides a ≥50% reduction in LDL-C.
Patients at lower risk may be managed with a more modest LDL-C reduction
approach, with the recognition there will be some variation in response
according to the dose provided. The benefits of therapy must be considered in
the context of safety to avoid possible adverse events in all patients.
HDL-C has been the subject of intense epidemiological and clinical
investigation. HDL-C levels are influenced by lifestyle factors, such as diet,
exercise, alcohol intake, obesity, and smoking, as well as specific drug
therapy (e.g., diuretics and anabolic steroids). Of these factors, exercise,
estrogens, and alcohol increase HDL-C, yet the possible benefits of these
influences are unproven and not endorsed as preventive strategies. Moreover,
recent clinical trials, including the use of niacin and CETPis, on raising
HDL-C have been proven to have limited clinical usefulness. Interest in
clinical trials with niacin preparations dates back >40 years to the results
of the Coronary Drug Project. As a therapeutic intervention, niacin has multiple effects on serum lipoproteins
(including LDL-C, TGs, and HDL-C), yet recent trials, including the
Atherothrombosis Intervention in Metabolic syndrome with Low-HDL and High
Triglycerides (AIM-HIGH) and Heart Protection Study 2-Treatment of HDL to
Reduce the Incidence of Vascular Events (HPS2 THRIVE) revealed no
benefit outcomes and the potential for harm.
More recently, the option of using CETPis to raise HDL-C have been
studied. The prototype agent, torcetrapib, increased HDL-C by >50%, together with 15% to 20% lowering
of LDL-C, yet the investigation was terminated early due to an increase in
cardiovascular and overall mortality in the treatment group. It is likely that
an off-target effect on electrolytes and blood pressure elevations produced
untoward toxicity. An alternate approach to CETP inhibition in the form of
dalcetrapib, which had no apparent off-target effects similar to torcetrapib, had
more modest effects on HDL-C and LDL-C. The early outcomes study, dal-OUTCOMES,
was terminated due to clinical futility. The most recent attempt to demonstrate
efficacy with a CETPi used evacetrapib, which had a potent effect on HDL-C and
other presumably beneficial effects on other lipid biomarkers; LDL-C and
lipoprotein(a) [Lp(a)] showed no evidence of benefit in the primary endpoint of
vascular events. However, another outcome trial that used anacetrapib, which
had similar dramatic effects on HDL-C, LDL-C, and Lp(a), showed modest but
significant benefit. Taken together, these trials suggest that CTEP inhibition
and drugs to raise HDL-C is not a major pathway to improving cardiovascular outcomes. However,
although the implications of HDL-C as a target of treatment remains unresolved,
the usefulness of HDL-C as an important predictor of cardiovascular risk
remains unchallenged.
TGs are important plasma lipids found in varying concentrations in all
plasma lipoproteins. The relationship between plasma TGs and CHD is still
unclear due to the lack of specific randomized clinical trials demonstrating
benefit outcomes. Recent epidemiological analyses suggest that elevated TGs, or
so-called remnant lipoproteins, are a contributor to residual risk of ASCVD.
Elevations in TGs in the range of 200 to 500 mg/dL should be interpreted as a
component of residual risk, and may obscure our interpretation of LDL-C values
from laboratory assessments. In this
context, using advanced diagnostic parameters
of apolipoprotein B or LDL particles (via nuclear magnetic resonance) is
comparable in association with clinical outcomes to assess risk for CVD when
questions arise on standard laboratory analyses.
Patients with genetic disorders of lipid metabolism or familial hyper-cholesterolemia
(FH) are at particularly high risk for coronary artery disease. These
individuals present with premature atherosclerotic heart disease, a strong
family history of coronary disease, and represent a significant clinical
challenge to healthcare providers. The prevalence of HeFH, which is a
heterozygote FH with baseline LDL-C levels ≥190 mg/ dL, in the general
population is believed to occur in 1 in 250 individuals based on recent
population data. Such patients are a priority treatment group according to the
current treatment guidelines. The introduction of PCSK-9 inhibitors and the
attendant science on LDL receptor regulation have provided significant insights
into epidemiological and clinical considerations in addressing the challenges of
FH. FH often remains underdiagnosed and undertreated until after a primary
coronary event. Historically, the treatment approach has been limited to a
combination of statins and other oral therapies or plasma apheresis. The advent
of newer treatment strategies, including mipomersen lomatipide, and PCSK-9
inhibitors (evolocumab and alirocumab), hold much promise for this patient population.
| FIG 16.1 Metabolic Syndrome. CHD, Coronary heart disease; HDL-C, high-density lipoprotein cholesterol; IGT, impaired glucose tolerance; LDL-C, low-density lipoprotein cholesterol; NIDDM, non–insulin-dependent diabetes mellitus; VLDL-C, very low-density lipoprotein cholesterol. |
MANAGEMENT AND THERAPY
Therapeutic Lifestyle
The proof of efficacy of statin and other drugs is built on effective lifestyle modification, such as healthy diet and physical activity, which are generally a part of randomized trials combined with these agents (Fig. 16.3). Patients should receive dietary counseling by a trained physician, nurse, or nutritionist. As in previous clinical recommendations, the recent AHA/ACC guidelines continue to emphasize the importance of lifestyle modification (i.e., adhering to a heart healthy diet, regular exercise habits, avoidance of tobacco products, and maintenance of a healthy weight) as a critical component of health promotion and ASCVD risk reduction before and in concert with cholesterol-lowering drug therapies (Fig. 16.3). The 2013 Lifestyle Management Work Group Guideline for lifestyle recommendations for healthy adults identified patterns of nutrition rather than specific diets such as the Dietary Approach to Stop Hypertension (DASH) or Mediterranean diets. These “patterns” include an emphasis on intake of fruits, vegetables, and whole grains. Sources of proteins should include low-fat dairy products, poultry, fish, and legumes, as well as limited intake of sweets, sugar-sweetened beverages, red meats, and overall calorie intake from saturated fat. Plant stanols/sterols (2 g/day) and up to 25 mg of soluble fiber have been suggested to aid in lowering LDL-C, either alone or in conjunction with appropriate pharmacotherapy.
 |
| FIG 16.2 Algorithm for Management of Lipid Goals. CAD, Coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. (Reused with permission from Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129[25 Suppl 2]:S1–45.) |
Physical activity is addressed in the 2008 Physical Activity Guidelines for Americans as a key to healthy aging. Although the 2013 AHA/ACC Lifestyle Management Guideline suggest 2.5 hours per week of moderate intensity exercise and promote physical activity, an individual approach should be outlined to support as much physical activity as abilities and conditions allow.
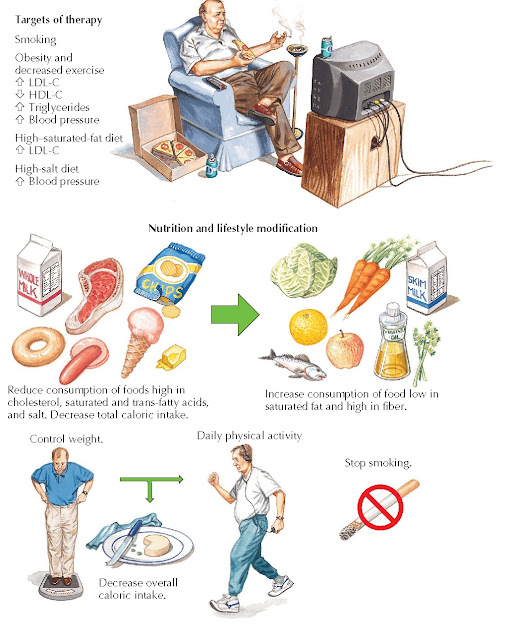 |
| FIG 16.3 Nonpharmacological Therapy for Management of Lipid Goals. HDL-C, High-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. |
Drug Therapy
Although all treatment options are not considered as first-line
treatment, there are various options that affect LDL-C and TG levels through
different mechanisms of action (Fig. 16.4). The 2013 ACC/AHA practice
guidelines provide an evidence-based drug treatment framework for treatment of
LDL-C and reducing cardiovascular risk. These recommendations are based on a
comprehensive review of randomized clinical trials of statins. They emphasize the
intensity of statin treatment based on the risk of the patient as an initial
approach to patient care. Highintensity treatment with statins to achieve an
LDL-C reduction of ≥50% and moderate intensity to achieve a reduction of 30% to
50% in LDL-C is recommended based on the risk group of the patients. The
decision to treat and the amount of
LDL-C reduction is based on the baseline risk
of the patient within the four treatment groups. Shared decision making with
the individual patient is emphasized (see Fig. 16.2).
Although the aggregate of randomized clinical trials with statins suggest
a graded and direct association between treated LDL-C and reduced
cardiovascular events, the available data do not support specific targets for
treatment goals. However, it is believed that even in patients who are
currently managed by effective doses of statins, additional cardiovascular
events might be reduced by more aggressive LDL-C lowering. The recent results
of the ezetimibe plus statin IMProved Reduction of Outcomes: Vytorin Efficacy
International Trial (IMPROVE- IT) showed a modest but significant reduction in
cardiovascular events with addition of this nonstatin therapy.
 |
The PCSK-9 inhibitors may introduce important options for patient
management because they share the ability to increase LDL-C receptor activity
with statins and produce dramatic reductions in LDL-C. Added to statin therapy,
they can reduce LDL-C by an additional 50% to 60% and produce treatment levels
well below current levels that can generally be achieved with statins alone.
The results of large outcome studies show great promise, and a recent study
level meta-analysis suggested improved all-cause mortality and fewer myocardial
infarction events, as well as a possible reduction in cardiovascular mortality.
Potentially serious adverse events associated with PCSK-9 inhibition appear to
be low.
Despite the extensive data supporting the safety and efficacy of statins
to lower LDL-C, many patients may not tolerate statin therapy in doses
necessary to achieve optimal outcomes. This may be in part due to patient
concern about drug safety and the poor understanding of patients about the
risks and benefits of statins. Medication adherence is often a multi-faceted
issue, and interventions to improve statin adherence must be individualized to
the patient. “De-prescribing” in older adults based on considerations of
polypharmacy, as well as defined risk and benefit may also be appropriate.
However, for all patients, the decision to treat should be accompanied by
information to support a clear understanding for the patient to appreciate the benefits and risks of the options of
their care.





