CENTRAL DIABETES
INSIPIDUS
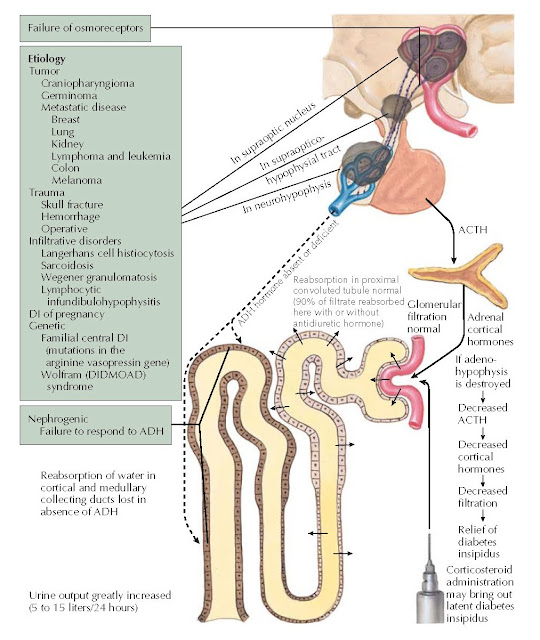
Diabetes insipidus (DI) literally means a large volume of urine (diabetes) that is tasteless (insipid). Central DI is characterized by a decreased release of antidiuretic hormone (ADH; vasopressin), resulting in polydipsia and polyuria. ADH deficiency may be a result of disorders or masses that affect the hypothalamic osmoreceptors, the supraoptic or paraventricular nuclei, or the superior portion of the supraopticohypophyseal tract. Approximately 90% of the vasopressinergic neurons must be destroyed to cause symptomatic DI. Because the posterior pituitary gland stores but does not produce ADH, damage by intrasellar pituitary tumors usually does not cause DI. The most common causes of central DI are trauma (e.g., neurosurgery, closed-head trauma), primary or metastatic tumors, and infiltrative disorders. Central DI can be exacerbated by or first become apparent during pregnancy, during which ADH catabolism is increased by placental hyperproduction of the enzyme cysteine aminopeptidase (vasopressinase).
Persons with central DI typically
have a sudden onset of polyuria and thirst for cold liquids. They usually wake
multiple times through the night because of the need to urinate and drink fluids often ice cold water
from the refrigerator. When seen in the outpatient clinic, these patients
usually have a large thermos of ice water by their side. The degree of polyuria
is dictated by the degree of ADH deficiency urine output
may range from 3 L/day in mild partial DI to more than 10 to 15 L/day in severe
DI. In patients with concurrent anterior pituitary failure, secondary adrenal
insufficiency is associated with decreased glomerular filtration (associated with
decreased blood pressure, cardiac output, and renal blood flow), leading to
decreased urine output. Glucocorticoid deficiency also increases ADH release in patients
with partial DI. These effects are reversed when glucocorticoid replacement is
administered and DI is “unmasked,” resulting in the rapid onset of polyuria.
 |
| Plate 1-27 |
Neurosurgery in the sellar region
(either by craniotomy or transsphenoidal routes) or blunt head trauma that
affects the hypothalamus and posterior pituitary may result in DI. As many as
50% of patients experience transient central DI within 24 hours of pituitary
surgery; it resolves over several days. With the minimally invasive endoscopic
transnasal transsphenoidal approach to pituitary surgery, the rate of
postoperative permanent DI is less than 5%; however, with transcranial
operations and with larger tumors that have hypothalamic involvement (e.g.,
craniopharyngioma), up to 30% of patients develop permanent DI. Damage to the
hypothalamus by neurosurgery or trauma often results in a triphasic response:
(1) an initial polyuric phase related to
decreased ADH release because of axon shock
and lack of action potential propagation beginning
within 24 hours of surgery and lasting approximately 5 days; (2) an
antidiuretic phase (days 6–12), during which stored ADH is slowly released from
the degenerating posterior pituitary, and hyponatremia may develop if excess
fluids are administered; and (3) a permanent DI after the posterior pituitary
ADH stores are depleted. In 10% to 25% of all patients who undergo pituitary
surgery, only the second of these three phases is seen, and DI never develops
because surgical trauma only damages some of the axons; this results in
inappropriate ADH release, but the intact axons continue to function and
prevent the onset of DI. However, this transient, inappropriate release of ADH
can have serious consequences, with marked hyponatremia that peaks
approximately 7 days after surgery and that may be associated with head-aches,
nausea, emesis, and seizures. This sequence of events can be avoided by
advising the patient to “drink for thirst only for the first 2 weeks” after a
pituitary operation.
Primary (e.g., craniopharyngioma,
germinoma) or metastatic (e.g., breast, lung, kidney, lymphoma, leukemia,
colon, or melanoma) disease in the brain can involve the hypothalamic–pituitary
region and lead to central DI. Polyuria and polydipsia may be the presenting
symptoms of metastatic disease.
Patients with Langerhans cell
histiocytosis are at high risk of developing central DI because of
hypothalamic–pituitary infiltration. Additional infiltrative disorders that may
cause central DI include sarcoidosis, Wegener granulomatosis, and autoimmune
lymphocytic infundibulohypophysitis.
Familial central DI is an autosomal
dominant disorder caused by mutations in the arginine vasopressin gene, AVP.
The incorrectly folded and mutant AVP prohormone accumulates in the endoplasmic
reticulum of the supraoptic and paraventricular nuclei and results in cell
death. Thus, children with autosomal dominant disease progressively develop AVP
deficiency, and symptoms usually do not appear until several months or years
after birth. Indeed, the posterior pituitary bright spot on magnetic resonance imaging (see Plate 1-8) is
present early and then slowly disappears with age and advancing damage to the
vasopressinergic neurons.
Wolfram (DIDMOAD [diabetes
insipidus, diabetes mellitus, optic atrophy, and deafness]) syndrome is
characterized by central DI, diabetes mellitus, optic atrophy, and deafness.
Diabetes mellitus typically occurs before central DI. This syndrome is usually
inherited in an autosomal recessive manner with incomplete penetrance, although
evidence suggests that there is another form of the disorder that may be caused
by mutations in mitochondrial DNA.




