SURGICAL TREATMENT OF
HYDROCEPHALUS
Transient
hydrocephalus can be temporarily treated with an external ventriculostomy or
lumbar drain. These temporary drainage systems allow constant monitoring of the
amount and character of CSF drainage, which can be quite helpful in patients
with a limited neurologic examination. For obstructive hydrocephalus, the CSF
diversion must occur above the blockage. In preterm infants, temporary
treatment of symptomatic hydrocephalus is achieved with a ventriculosubgaleal
shunt that drains the CSF into a subgaleal pocket or into a ventricular access
device that has a reservoir to tap to remove CSF. Once the preterm infant
achieves an adequate size, a more permanent CSF diversion procedure is
performed, if needed.
Endoscopic
procedures for CSF diversion include endoscopic third ventriculostomy (ETV),
cyst fenestration, choroid plexus coagulation, and other procedures. The
success of these procedures depends on multiple factors, including patient
selection and specific anatomic details. The primary benefit of endoscopic
procedures is the avoidance of implantation of shunt components that may later
malfunction, become infected, or induce shunt dependence. Endoscopic procedures
for CSF diversion can have late failure, and all patients after endoscopic
procedures continue to require chronic neurosurgical supervision similar to
patients with shunts.
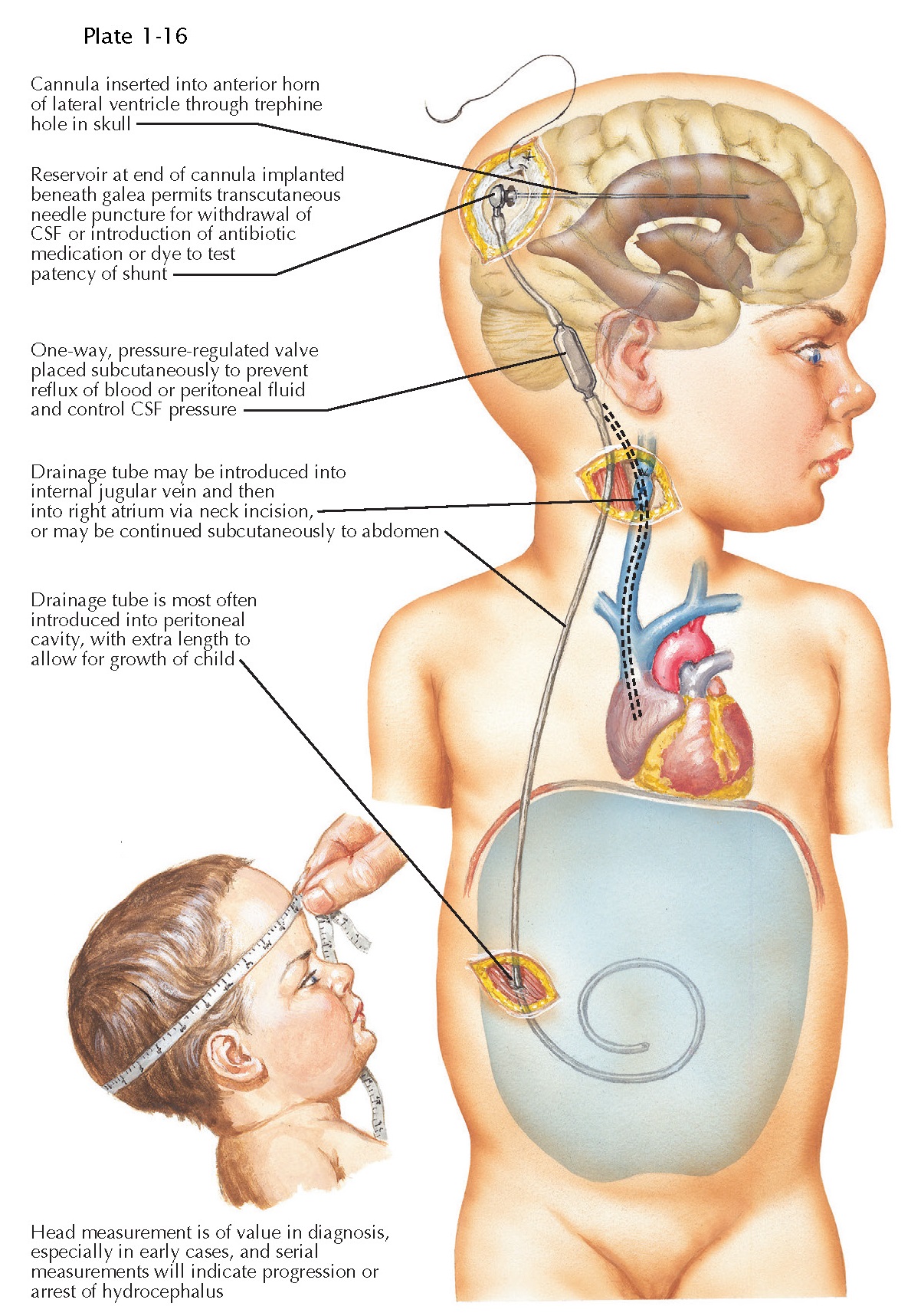
The
most common shunt system used is a ventriculoperitoneal shunt with a valve.
Shunt components are made from Silastic material, and some are
antibiotic-impregnated to decrease the risk of infection. The ventricular
catheter tip is targeted to the frontal horn of a lateral ventricle from either
a frontal or parieto-occipital trajectory. As the catheter exits the skull in
the subcutaneous space, it is connected to a valve. Some surgeons use an
intervening reservoir. The goal of the valve is to minimize overdrainage and
subsequent collapse of the ventricular system and formation of life-threatening
subdural hematomas. Various types of valves have been devised; none among them
has been proved superior in a well-designed multicenter trial. Shunt tubing can
also contain a valve at the distal tip. The subcutaneous distal shunt tubing is
inserted into the peritoneal cavity, where the peritoneum absorbs the CSF back
into systemic veins. Adequate tubing is placed in infants to decrease the
chance that a lengthening procedure will be required. Alternate distal tubing
sites include the right atrium or the pleural cavity. Lumboperitoneal shunts
are used in select patients. Occasionally, it is necessary to obtain CSF from a
patient with a shunt or to inject antibiotics or chemotherapy into the
ventricular system instead of via a lumbar puncture. Rarely, contrast material
may also be injected to identify loculations within the ventricular cavity. Any
manipulation of a shunt by a non-neurosurgeon
should be performed only in direct collaboration with a neurosurgeon.
The
long-term success of the CSF diversion procedure depends upon the continued
patency of the shunt or endoscopic opening. Failure of an endoscopic
fenestration can lead to the same symptoms and signs of neurologic decline as a
shunt failure. Once shunted, patients who may have previously absorbed a portion
of their CSF may become completely dependent on the shunt for CSF diversion.
The clinical presentation of a patient with failure of the CSF diversion
procedure may or may not mimic the symptoms at the
time of the diagnosis of hydrocephalus and initial treatment. The symptoms and
signs of failure and period of illness may depend on the type of failure, the
etiology of hydrocephalus and the patient’s age. The most common cause of shunt
malfunction is proximal catheter occlusion. Many patients with CSF diversion
failure will present with recurrence of ventriculomegaly. Of importance, 10% to
20% of children presenting with a shunt malfunction will have no apparent
change in the ventricular size compared with a baseline
imaging study.