SEMEN ANALYSIS AND SPERM MORPHOLOGY
Although not a true measure of fertility, the semen analysis, if abnormal, suggests that the probability of achieving fertility is lower than normal. For a male infertility evaluation, two semen analyses, performed with 2 to 3 days of sexual abstinence, are sought due to the large biologic variability in semen quality. Lubricants should be avoided and the specimen kept at body temperature during transport.
Normal values have been defined for the human semen analysis by expert
consensus (World Health Organization). Fresh semen is a coagulum that liquefies
from 5 to 30 min after ejaculation. After liquefaction, semen viscosity is
measured and should not show any stranding. Ejaculate volume should be at least
1.5 mL, as smaller volumes may not sufficiently buffer against vaginal acidity.
Although most commonly a consequence of collection error, low ejaculate volume
may also indicate retrograde ejaculation, ejaculatory duct obstruction, or
androgen deficiency. Sperm concentration should be 20 million sperm/mL. Reasons
for low sperm concentrations can include medications, exposures, systemic
disease, hormonal disorders, varicocele, unilateral blockage, and genetic
syndromes. Sperm motility is assessed in two ways: the proportion of all sperm
that are moving and the quality of sperm movement. A normal value for sperm
motility is 50% motile along with an average quality or progression score. The
causes of low sperm motility, the most common semen analysis finding, are myriad
and often reversible.
Recently, however, there has been debate concerning precisely which semen
analysis values are to be considered “normal,” as controlled studies of fertile
and infertile couples suggested other thresholds may be more appropriate, and
sperm production is known to be susceptible to wide individual, geographic, and
seasonal variation. When assessing semen quality, it is important to realize
that spermatogenesis takes 60 to 80 days to complete, so that an individual
semen analysis reflects biologic influences occurring 2 to 3 months prior.
Like-wise, medical or surgical therapy directed at improving semen quality will
take several months to become manifest in improved semen quality.
Although seasonal variation exists, sperm production is a rapid and
relatively constant process in humans. This is in part due to the anatomy of
sperm production within the seminiferous tubules. A cycle of spermatogenesis
involves the division of primitive germ cells into later germ cells. Many
cycles of spermatogenesis coexist within the germinal epithelium at any one
time, and they are described morphologically as stages. In addition, there is
also a specific organization of spermatogenic cycles within the tubular space,
termed spermatogenic waves. Although well described in other mammals, the exact
configuration of spermatogenic waves in humans has been debated. The best evidence
suggests that human spermatogenesis exists in a spiral or helical cellular
arrangement that ensures that sperm production is a continuous and not a
pulsatile process.
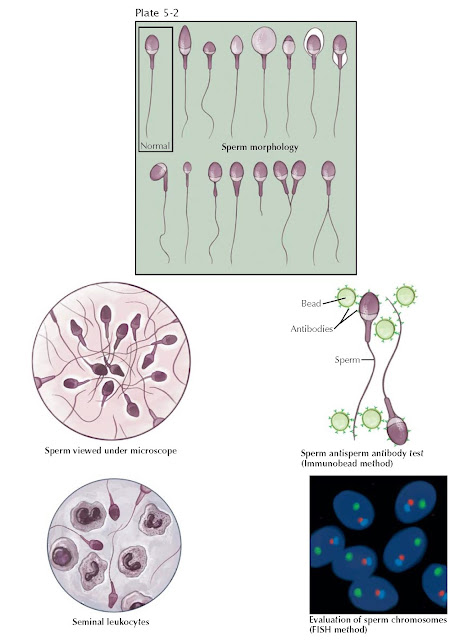
The formal evaluation of sperm shape is termed morphologic assessment.
Several descriptive systems exist to evaluate morphology, and within each
system, sperm are designated normal or abnormal based on specific size criteria.
Although it is essentially judging a book by its cover, since the late 1980s it
has been believed that sperm morphology may correlate with a man’s fertility
potential as reflected by in vitro fertilization (IVF). In general, the percentage of sperm with normal morphology has the
greatest discriminatory power among all descriptors of semen quality in
distinguishing fertile from infertile semen, although no particular value is
diagnostic of fertility. Sperm morphology can be altered by toxic and
occupational exposures, varicocele, fevers, medications, and systemic disease.
It appears that sperm morphology is a sensitive indicator of overall testicular
health, because the sperm morphologic characteristics are determined during
spermatogenesis.
Other fertility assays can evaluate whether the seminal environment is abnormal, which may contribute to male infertility. Two such tests include an evaluation for excessive semen leukocytes (leukocytospermia) and testing for antisperm antibodies that can inhibit sperm transport through the female reproductive tract and impair sperm–egg interaction at fertilization. Sperm genetics can also be directly assessed for chromosomal normalcy with in situ hybridization techniques. These tests can complement the routine semen analysis in the male evaluation and better estimate the chances of fertility.