LIPIDS
Lipids have three major roles in cells. First, they provide an important form of energy storage. Second, and of great importance in cell biology, lipids are the major components of cell membranes. Third, lipids play important roles in cell signaling, both as steroid hormones (e.g., estrogen and testosterone) and as messenger molecules that convey signals from cell surface receptors to targets within the cell. The simplest lipids are fatty acids, which consist of long hydrocarbon chains, most frequently containing 16 or 18 carbon atoms, with a carboxyl group (COO–) at one end (Figure 2.8). Unsaturated fatty acids contain one or more double bonds between carbon atoms; in saturated fatty acids all of the carbon atoms are bonded to the maximum number of hydrogen atoms. The long hydrocarbon chains of fatty acids contain only nonpolar C—H bonds, which are unable to interact with water. The hydrophobic nature of these fatty acid chains is responsible for much of the behavior of complex lipids, particularly in the formation of biological membranes.
 |
Figure 2.8 Structure of fatty
acids Fatty acids consist of long
hydrocarbon chains terminating in a carboxyl group (COO–). Palmitate and stearate are saturated fatty
acids consisting of 16 and 18 carbons, respectively. Oleate is an unsaturated 18-carbon fatty acid
containing a double bond between carbons 9 and 10. Note that the double bond introduces a kink in
the hydrocarbon chain.
Fatty acids are stored in the form of triacylglycerols (also called triglycerides or fats), which consist of three fatty acids linked to a glycerol molecule (Figure 2.9). Triacylglycerols are insoluble in water and therefore accumulate as fat droplets in the cytoplasm. When required, they can be broken down for use in energy-yielding reactions (see Chapter 3). It is noteworthy that fats are a more efficient form of energy storage than carbohydrates, yielding more than twice as much energy per weight of material broken down. Fats therefore allow energy to be stored in less than half the body weight than would be required to store the same amount of energy in carbohydrates a particularly important consideration for animals because of their mobility.
 |
Figure 2.9 Structure of triacylglycerols
Triacylglycerols (fats)
contain three fatty acids joined to
glycerol. In this example, all three fatty acids are palmitate, but
triacylglycerols often contain a mixture
of different fatty acids.
Phospholipids, the principal components of cell membranes, consist of two fatty acids joined to a polar head group (Figure 2.10). In the glycerol phospholipids, the two fatty acids are bound to carbon atoms in glycerol, as in triacylglycerols. The third carbon of glycerol, however, is bound to a phosphate group, which is in turn frequently attached to another small polar molecule, such as ethanolamine, choline, serine, or inositol. Sphingomyelin, the only nonglycerol phospholipid in cell membranes, contains two hydrocarbon chains linked to a polar head group formed from serine rather than from glycerol. All phospholipids have hydrophobic tails (consisting of the two hydrocarbon chains), and hydrophilic head groups (consisting of the phosphate group and its polar attachments). Consequently, phospholipids are amphipathic molecules, part water-soluble and part water-insoluble. This property of phospholipids is the basis for the formation of biological membranes, as discussed later in this chapter.
 |
Figure 2.10 Structure of
phospholipids Glycerol phospholipids contain two fatty acids
joined to glycerol. The fatty acids may be different from each other. The third carbon of glycerol is joined
to a phosphate group
(forming phosphatidic acid),
which in turn is frequently joined to another small polar molecule (forming phosphatidylethanolamine, phosphatidylcholine,
phosphatidylserine, or phosphatidylinositol). In sphingomyelin, two hydrocarbon chains are bound to a polar head group formed from
serine instead of glycerol.
In addition to phospholipids, many cell membranes contain glycolipids and cholesterol. Glycolipids consist of two hydrocarbon chains linked to polar head groups that contain carbohydrates (Figure 2.11). They are thus similar to the phospholipids in their general organization as amphipathic molecules. Cholesterol, in contrast, consists of four hydrocarbon rings rather than linear hydrocarbon chains (Figure 2.12). The hydrocarbon rings are strongly hydrophobic, but the hydroxyl (OH) group attached to one end of cholesterol is weakly hydrophilic, so cholesterol is also amphipathic.
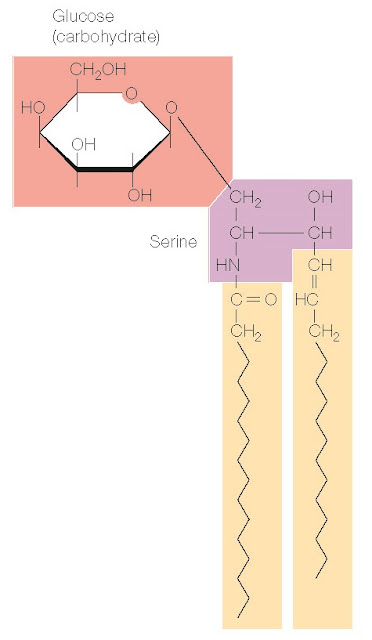 |
Figure 2.11 Structure of
glycolipids Two hydrocarbon chains
are joined to a polar head group formed
from serine and containing
carbohydrates (e.g., glucose).
In addition to their
roles as components of cell membranes, lipids function as signaling molecules,
both within and between cells. The steroid hormones (such as
testosterone and estrogens) are derivatives of cholesterol (see Figure 2.12).
These hormones are a diverse group of chemical messengers, all of which contain
four hydrocarbon rings to which distinct functional groups are attached.
Derivatives of phospholipids also serve as messenger molecules within cells,
acting to convey signals from cell surface receptors to intracellular targets
that regulate a wide range of cellular processes, including cell proliferation, movement, survival,
and differentiation (see Chapter 17).
 |
Figure 2.12 Cholesterol and steroid hormones Cholesterol, an
important component of cell
membranes, is an
|
amphipathic molecule
because of its polar hydroxyl group.
Cholesterol is also a precursor to the
steroid hormones, such as testosterone
and estradiol (a form of estrogen). The
hydrogen atoms bonded to the ring
carbons are not shown in this figure. |




