B‐CELLS RESPOND TO THREE DIFFERENT TYPES OF ANTIGEN
There are three main types of B‐cell that respond to infection by secreting antibodies that target specific classes of microbes, with the particular function of each B‐cell subset generally determined by their location. Follicular B‐cells (also called B2 cells) express highly specific monoreactive B‐cell receptors (BCRs), are present in the lymphoid follicles of the spleen and lymph nodes, and typically require T‐cells in order to generate high‐affinity antibodies, and to undergo class switching (Figure 7.21). However, as we shall discuss below, certain types of antigens (called T‐independent antigens) can promote B‐cell activation without the help of T‐cells. The antibodies thus formed are typically of low affinity and do not undergo class switching or somatic hypermutation but provide rapid protection from certain microorganisms and buy time for T‐dependent B‐cell responses to be made. Such rapid antibody responses are mediated by the “innate like” B‐cells; B1 and marginal zone (MZ) B‐cells, which express polyreactive BCRs that are of broad specificity and enable them to recognize multiple different kinds of evolutionarily conserved microbial antigens. In this way, they are similar to the Toll‐like receptors (TLRs) expressed on conventional innate immune cells. Indeed, innate‐like B‐cells also express TLRs and can be directly activated by PAMPs, act as APCs, and secrete cytokines, which places them at the interface between the innate and adaptive immune systems. Importantly, this innate‐like B‐cell response is positioned at strategic areas that are sensitive to microbial invasion, such as the skin, mucosa, and the marginal zone of the spleen, where the lymphatic and circulatory systems converge.
 |
Figure 7.21 Interaction between B‐cells and T‐cells. Scanning electron microscope analysis of a cognate B‐cell/T‐cell pair, embedded in 3‐D collagen matrix.
1. Type 1 thymus‐independent antigens
Certain antigens, such as
bacterial lipopolysaccharides, when present at a sufficiently high
concentration have the ability to activate a substantial proportion of the B‐cell pool polyclonally
(i.e., without reference to the antigen specificity of the surface receptor
hypervariable regions). They do this through binding to surface molecules, such
as TLRs as discussed in Chapter 1, which bypasses the early part of the biochemical pathway mediated by the
specific antigen receptor. At concentrations that are too low to cause
polyclonal activation through unaided binding to these mitogenic bypass
molecules, the B‐cell population with Ig
receptors specific for these antigens will selectively and passively focus them
on their surface, where the resulting high local concentration will suffice to
drive the activation process (Figure 7.22a).
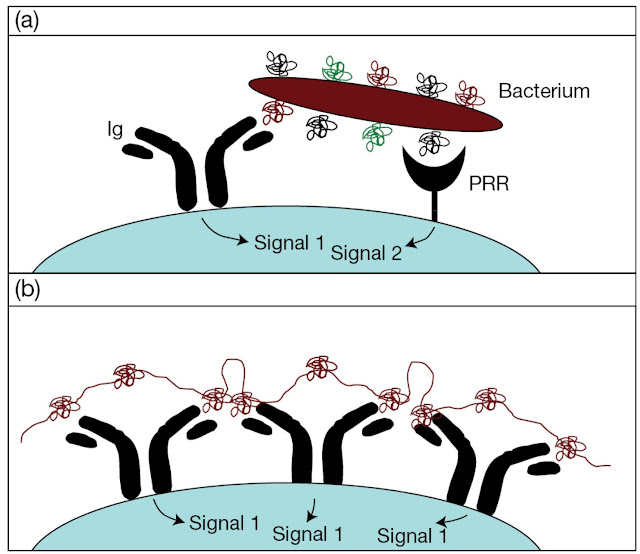 |
Figure 7.22 B‐cell recognition of (a) type 1 and (b) type 2
thymusindependent antigens. The complex gives a sustained signal to the B‐cell because of the long half‐life of this type of molecule.
2. Type 2 thymus‐independent
antigens
Certain linear antigens that
are not readily degraded in the body and that have an appropriately spaced, highly
repeating determinant–Pneumococcus polysaccharide, Ficoll, d‐amino acid polymers, and
polyvinylpyrrolidone, for example – are also thymus‐independent in their ability
to stimulate B‐cells directly without the
need for T‐cell involvement. Such
antigens persist for long periods on the surface of follicular DCs located at
the subcapsular sinus of the lymph nodes and the splenic marginal zone, and can
bind to antigen‐specific B‐cells with great avidity
through their multivalent attachment to the complementary Ig receptors that
they cross‐link (Figure 7.22b).
In general, the thymus‐independent antigens give
rise to predominantly low‐affinity IgM responses, some
IgG3 in the mouse, and relatively poor, if any, memory. Neonatal B‐cells do not respond well to
type 2 antigens and this has important consequences for the efficacy of
carbohydrate vaccines in young children.
This innate, T‐cell‐independent detection of
microbial antigen is mediated by two types of B‐cell: marginal zone (MZ) B‐cells and B1 B‐cells. MZ B‐cells are located in the
marginal zone of the spleen. This specialized area, located at the interface
between the circulatory and lymphatic system, acts as a type of filter for
blood‐borne pathogens and MZ B‐cells there constantly
monitor the circulating levels of PAMP. In contrast, B1 B‐cells are found in the skin and mucosal surfaces, areas continually under
siege from pathogens, and act as a rapid first line of defense against
microbial invasion. Importantly, activation of both of these innate B‐cell types by simultaneous
trigger of BCR and TLRs not only promotes a strong IgM and IgG3 response, but
also presents antigen to T‐cells, thus quickly
activating the adaptive immune response. Mice specifically deficient in B‐cell Myd88, an essential
signal transducer for TLRs, show strong defects in their ability to mount an
antibody‐mediated response to many
types of infection, suggesting an important role for intrinsic TLR signaling in
B‐cell function.
 |
Figure 7.23 T‐helper cells cooperate through protein carrier determinants to help B‐cells respond to hapten or equivalent determinants on antigens (Ag) by providing accessory
signals. (For simplicity we are ignoring the MHC component and
epitope processing in T‐cell recognition, but we won’t forget it.)
3. Thymus‐dependent antigens
The need for collaboration with T‐helper
cells
Many antigens are thymus‐dependent in that they
provoke little or no antibody response in animals that have been thymectomized
at birth and therefore have few T‐cells (Milestone 7.1). Such antigens cannot fulfill the molecular
requirements for direct stimulation: they may be univalent with respect to the
specificity of each determinant; they may be readily degraded by phagocytic
cells; and they may lack mitogenicity. If they bind to B‐cell receptors, they will
sit on the surface just like a hapten and do nothing
to trigger the B‐cell (Figure 7.23). Cast
your mind back to the definition of a hapten – a small molecule such as
dinitrophenyl (DNP) that binds to preformed antibody (e.g., the surface
receptor of a specific B‐cell) but fails to stimulate
antibody production (i.e., stimulate the B‐cell). Remember also that haptens become immunogenic when coupled to an
appropriate carrier protein. Building on the knowledge that both T‐ and B‐cells are necessary for
antibody responses to thymus‐dependent antigens (Milestone 7.1), we now know that the
carrier functions to stimulate T‐helper cells that cooperate with B‐cells to enable them to respond to the hapten by providing accessory
signals (Figure 7.23). It should also be evident from Figure
7.23 that, while one determinant on a typical protein antigen is
behaving as a hapten in binding to the B‐cell, the other determinants subserve a carrier function in recruiting T‐helper cells.
 |
Figure 7.24 T‐ and B‐cell interaction in a B‐cell follicle. Multiple Tcell (red) and B‐cell (green) pairs form at the T zone border within a
B‐cell follicle (arrowheads).
 |
Figure 7.25 B‐cell handling of a thymus‐dependent antigen and presentation to an activated T‐cell. Antigen captured by the
|
surface Ig receptor is internalized within an endosome, processed, and expressed on the surface of the B‐cell with MHC class II (see Figure 5.16). Co‐stimulatory signals through the CD40–CD40L (CD154) interaction are required for the activation of the resting B‐cell by the T‐helper cell. In addition to CD40L‐based co‐stimulation, helper T‐cells also provide additional stimulation to the B‐cell in the form of cytokines such as IL‐4. |
Antigen processing by B‐cells
The need for physical
linkage of hapten and carrier strongly suggests that T‐helpers must recognize the
carrier determinants on the responding B‐cell in order to provide the relevant accessory stimulatory signals.
However, as T‐cells only recognize
processed membrane‐bound antigen in association
with MHC molecules, the T‐helpers cannot recognize
native antigen bound simply to the Ig receptors of the B‐cell as naively depicted in Figure 7.23. All is not lost, however, as primed
B‐cells can present antigen to T‐helper
cells (Figure 7.24) – in fact, they work at much lower
antigen concentrations than conventional presenting cells because they can
focus antigen through their surface receptors. Antigen bound to surface Ig is
internalized in endosomes that then fuse with vesicles containing MHC class II
molecules with their invariant chain. Processing of the protein antigen then
occurs as described in Chapter 5 (see Figure 5.16)
and the resulting antigenic peptide is recycled to the surface in association
with the class II molecules, where it is available for recognition by specific
T‐helpers (Figure 7.25 and Figure 7.26).
The need for the physical union of hapten and carrier is now revealed; the
hapten leads the carrier to be processed into the cell, which is programmed to
make anti‐hapten antibody and,
following stimulus by the T‐helper‐recognizing processed
carrier, it will carry out its program and ultimately produce antibodies that
react with the hapten (is there no end to the wiliness of nature?).
 |
Figure M7.1.1 The antibody response to some antigens is thymus dependent and, to others, thymus independent. The
response to tetanus toxoid in neonatally thymectomized animals
could be restored by the injection of thymocytes
 |
Figure M7.1.2 The antibody response to a thymus‐dependent antigen requires two different lymphocyte
populations. Different populations of cells from a normal mouse histocompatible with the
recipient (i.e., of the same H‐2 haplotype)
were injected into recipients that had been X‐irradiated to destroy their own lymphocyte responses.
They were then primed with a thymus‐dependent antigen such as sheep red blood cells (i.e., an antigen that fails to
give a response in neonatally thymectomized mice; Figure M7.1.1) and examined
for the production of antibody after 2 weeks. The small
amount of antibody (Ab) synthesized by animals receiving bone marrow alone is due to the presence of thymocyte precursors in the
cell inoculum that differentiate in the intact thymus gland of the recipient.
Milestone 7.1 T–B collaboration for antibody production
In the 1960s, as the mysteries of the thymus
were slowly unraveled, our erstwhile colleagues pushing
back the frontiers of knowledge discovered that neonatal thymectomy in the
mouse abrogated not only the cellular rejection of skin grafts, but also the
antibody response to some but not all antigens (Figure M7.1.1). Subsequent investigations
showed that both thymocytes and bone marrow cells were needed for optimal antibody responses to such thymus‐dependent
antigens (Figure M7.1.2). By carrying
out these transfers with cells from
animals bearing a recognizable chromosome marker (T6), it became evident that
the antibody‐forming cells were derived from the bone marrow
inoculum, hence the nomenclature “T” for thymus‐derived lymphocytes and “B”
for antibody‐ forming cell precursors originating in the bone
marrow. This convenient nomenclature has stuck even though bone marrow contains
embryonic T‐cell precursors, as the immunocompetent T‐ and B‐cells differentiate in the
thymus and bone marrow, respectively.





