CARBONIC ANHYDRASE INHIBITORS
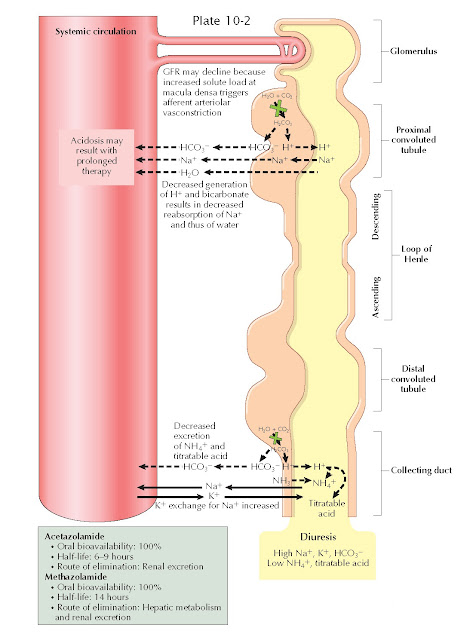
Carbonic anhydrase (CA) catalyzes the interconversion of carbon dioxide and water to bicarbonate (HCO ) ions and protons. There are multiple CA isoforms, which serve different functions in cells throughout the body. In the renal tubules, the epithelial cells involved in acid-base handling such as those in the proximal tubule, thick ascending limb, and the cortical collecting duct possess cytoplasmic CA-II and luminal membrane- bound CA-IV. Several other CA isoforms also appear to be present throughout the nephron, with their locations and functions still under active investigation.
As reviewed in Plates 3-21 and 3-22, CA plays crucial
roles in acid-base homeostasis and solute reabsorption. In the proximal tubule,
for example, membrane-bound CA-IV catalyzes the conversion of filtered
bicarbonate ions and secreted protons into carbon dioxide, which is reabsorbed
across the apical surface of proximal tubular cells. Cytoplasmic CA-II,
meanwhile, converts the reabsorbed carbon dioxide back into bicarbonate ions, which
are reabsorbed, and protons, which are secreted on the NHE-3 Na+/H+ exchanger. Meanwhile, in
more distal segments, cytoplasmic CA-II converts carbon dioxide to new
bicarbonate ions, which are reabsorbed, and protons, which are secreted and
bound in the tubular lumen to titratable acids and ammonia.
CA inhibitors (CAIs) are sulfonamide derivatives that
block the actions of both cytoplasmic and membrane-bound CA isoforms throughout
the nephron. As a result, CAIs cause an increased fraction of the filtered
bicarbonate to be excreted, rather than reabsorbed. In addition, these agents
impair protonation of titratable acids and ammonia in the distal nephron.
Because of these effects, the urine becomes inappropriately alkalotic, and
metabolic acidosis ensues.
In addition to their effects on acid-base equilibrium,
CAIs also impair proximal Na+ reabsorption, since operation of the NHE-3 exchanger depends on the
presence of protons in the cytoplasm of tubular epithelial cells. The
natriuretic effect of this action, however, is largely offset by upregulation
of distal Na+ reabsorption sites, such as the thick ascending limb, that possess
proton-independent Na+ transport mechanisms. More- over, the increased solute load delivered
to the macula densa stimulates afferent arteriolar vasoconstriction, reducing
the glomerular filtration rate.
Because CA is widely distributed through the body,
CAIs have multiple extrarenal effects that render them useful in other clinical
settings. In the ciliary processes of the eye, for example, CAIs reduce the
production of aqueous humor, making them effective agents in the treatment of
glaucoma.
COMMON AGENTS
The major CAIs are listed in the plate.
INDICATIONS
The most common indications for CAIs include:
·
Edema, but they are rarely given for this indication because other diuretic
classes are safer and more effective.
· Acute mountain sickness. At high altitudes, the low partial pressure of oxygen induces
hyperventilation. This response improves tissue oxygenation but is limited by
the ensuing respiratory alkalosis.
Acetazolamide can be used to induce metabolic acidosis
and further increase ventilation.
· Open-or closed-angle
glaucoma. As described above, CAIs reduce
production of aqueous humor in the anterior chamber of the eye. Methazolamide
is preferred for this indication because of its long half-life. In closed-angle
glaucoma, CAIs can be used as a temporizing measure until definitive treatment
is available.
ADVERSE EFFECTS
The major adverse effects of CAIs include:
·
Metabolic acidosis
· Nephrolithiasis, owing to urine alkalinization
· Increased serum ammonia
concentration, secondary to impaired renal
excretion, which may cause encephalopathy in patients with cirrhosis
· Bone marrow suppression
· Skin toxicity
· Confusion, drowsiness, and
paresthesia, secondary to central nervous system effects
CAIs increase K+ excretion in several ways. First, the increased Na+ load that reaches the distal
nephron creates a negative intraluminal
charge as it is reabsorbed, which promotes K+ secretion through apical renal outer medullary potassium (ROM-K) channels. Second, the increased HCO3− load
that reaches the distal
nephron also generates a negative intraluminal charge, which promotes K+ secretion for the same reasons. Finally, the increased urine flow through the distal nephron promotes K+ secretion
through flow-sensitive maxi-K channels.
Such kaliuresis, however,
rarely leads to hypokalemia because metabolic acidosis stimulates K+ efflux from cells in exchange for H+ influx.