UROLITHIASIS
Renal stones are common, with lifetime prevalence estimates as high as 15%. Historically, men are affected more often than women, with a ratio of 2 or 3:1, although recent evidence suggests the gender gap may be closing. Stones can form at any age, but most occur in adults between 30 and 60 years of age. The clinical and economic impact of stone disease is substantial, with an estimated $2 billion spent in the United States in 2000.
Bladder stones are also associated with significant
morbidity but occur far less frequently than renal stones. Because the causes
of renal and bladder stones are distinct, their associated symptoms,
treatments, and prevention strategies are considered separately.
RENAL STONES
Pathogenesis. The majority
of renal stones (80%) are calcium-based, most frequently calcium oxalate and
less commonly calcium phosphate. Other less common stone compositions are uric
acid, struvite, and cystine. When stone-forming salts reach a urinary
concentration that exceeds the point of equilibrium between dissolved and
crystalline components, crystallization will occur. Although certain chemicals
in the urine can delay stone formation, there is a concentration of
stone-forming salts above which crystallization becomes inevitable. Thus
factors that increase the propensity for stone formation do so by reducing
urine volume, increasing the quantity of stone-forming salts, or decreasing the
quantity of crystallization inhibitors.
The process by which crystal formation leads to stone
formation remains incompletely understood. Recent evidence, however, suggests
that routine calcium oxalate stones originate on calcium phosphate deposits,
known as Randall plaques, that are located at the tips of renal papillae and
act as niduses for crystal overgrowth.
Risk Factors. Calcium stones
are associated with a variety of genetic, environmental, and dietary risk
factors. Elevated urinary calcium levels, one of the most common causes of
calcium stones, can occur in the setting of increased bone resorption,
intestinal hyperabsorption of calcium, or impaired renal tubular reabsorption
of calcium. Elevated urinary oxalate levels, either dietary or the result of
enhanced intestinal oxalate absorption, increase the urinary saturation of
calcium oxalate and promote stone formation.
Depressed urinary citrate levels, often idiopathic but
in some cases associated with systemic acidosis or hypokalemia, are also
associated with an increased risk of calcium stones because citrate is an
important inhibitor of stone formation. Finally, elevated urinary uric acid
levels promote calcium oxalate stone formation and are associated with
excessive intake of animal protein, conditions that lead to
overproduction/overexcretion of uric acid (e.g., Lesch-Nyhan syndrome), or use
of uricosuric medications.
Noncalcium stones are also associated with specific
metabolic, genetic, and infectious disorders. Uric acid stones primarily occur
in the setting of overly acidic urine, in which uric acid crystallizes. These stones are more common
among patients with insulin resistance and type II diabetes mellitus, in whom
production and excretion of ammonia in the renal proximal tubule is impaired,
leading to insufficient buffering of protons in urine. Magnesium ammonium
phosphate (struvite) stones, in contrast, occur in the setting of overly
alkaline urine, in which struvite and calcium carbonate precipitate. These stones primarily occur in patients
who have urinary tract infections with urea splitting bacteria, such as Proteus, Pseudomonas, Klebsiella,
and Staphylococcus. The hydrolysis of urea produces high concentrations
of ammonia, which buffers protons. Incorporation of bacteria into these stones
may cause chronic infections. Finally, cystine stones occur because of an
inherited disorder of amino acid transport in which proximal tubular reabsorption of dibasic amino acids (cystine,
lysine, ornithine, arginine) is impaired, leading to high urinary
concentrations. Because cystine is poorly soluble in urine, it crystallizes and
forms stones at relatively low urinary concentrations.
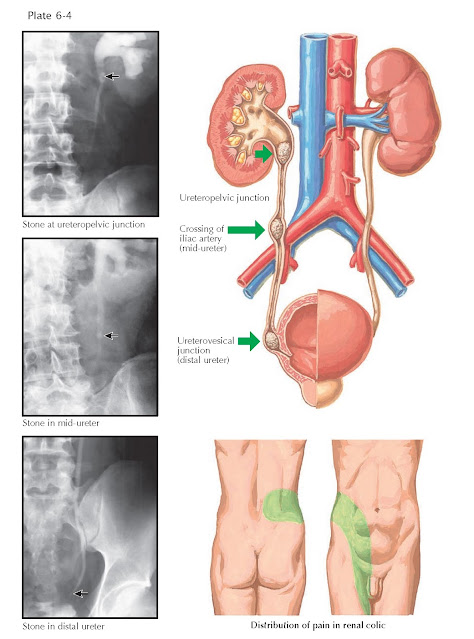
Presentation and Diagnosis. The symptoms associated with renal stones depend on their location.
Typically, stones located within the calyces are asymptomatic. When these
stones become detached and are propelled down the narrow ureter, however, they
frequently become impacted. Stones generally become lodged in the narrowest
portions of the ureter, which are located at the ureteropelvic junction, the
crossing of the iliac vessels, and the ureterovesical junction (see Plate 6–4).
The first sign of a stone in the ureter is often the acute onset of severe flank
pain. The stone obstructs urine outflow from the kidney, and the acute increase
in renal pelvic pressure causes distention of the collecting system and
stretching of the renal capsule, producing pain that classically starts in the
flank and radiates to the ipsilateral groin. For reasons that are incompletely
understood, the pain of a ureteral stone is typically intermittent, rather than
constant. Other symptoms include nausea, vomiting, and gross or microscopic
hematuria. This constellation of symptoms is known as renal colic.
Occasionally, the movement of a stone into the ureter can be associated with
obstruction and infection, culminating in pyelonephritis (see Plate 5–5) and/or
sepsis. In this situation, urgent relief of obstruction is required to
decompress the collecting system and allow antibiotics to be excreted into the urine.
Most renal stones can be detected on plain abdominal
radiographs because of their calcium content, although calcium-poor stones such
as pure uric acids stones are radiolucent. The primary diagnostic imaging tool,
however, is non–contrast enhanced CT, which has a 98% sensitivity for the
detection of stones. If intravenous contrast is administered and excreted into
the urine collecting system, stones may be obscured, since both stones and
contrast have high attenuation.
If a stone is identified, microscopic analysis of urine
may be helpful to determine stone composition, as characteristic crystals are
sometimes seen.
 |
| MAJOR SITES OF RENAL STONE IMPACTION |
Treatment. The treatment
of a renal stone depends on its size, location, and associated symptoms. The
majority of stones that enter the ureter will pass on their own, given enough
time. The likelihood of spontaneous stone passage is higher for stones that are
small and/or located in the distal ureter. The time interval to stone passage
is variable, and intermittent episodes of pain may accompany stone transit.
Once they reach the bladder, most stones can easily be voided, as the lumen of
the urethra is much larger than that of the ureter.
Medications such as calcium channel blockers and -receptor antagonists have been shown to influence
ureteral contractility and promote stone passage, thereby increasing the
likelihood of spontaneous passage, reducing the time interval to passage, and
decreasing the need for pain medication. A trial of these medications is
indicated in patients with ureteral stones less than 1 cm in size, particularly
if they are located in the lower part of the ureter at the time of diagnosis.
When a ureteral stone fails to pass or is too large to pass, or when pain becomes intolerable, surgical
intervention is warranted. Many small to moderate-sized stones can be treated
noninvasively, using shock waves focused on the stone under fluoroscopic or
ultrasound guidance (extracorporeal shockwave lithotripsy, see Plate 10-12).
Repeated application of focused shock-waves causes stone fragmentation, which
enables painless passage of fragments in the urine. Alternatively, stones can
be removed using a ureteroscope (see Plate 10-33), which is passed through the urethra and bladder up to the site
of the stone in the ureter. The stone is then fragmented with a laser or other
device inserted through the working channel of the ureteroscope.
For large and/or complex stones, such as staghorn
calculi (which occupy all or a large portion of the collecting system), a large
endoscope is introduced percutaneously into the kidney through a small incision
in the flank. The stone is then fragmented and the fragments removed (percutaneous nephrostolithotomy, see Plate 10-13).
Prognosis. After a first
renal stone is diagnosed, there is a nearly 50% likelihood of recurrence over
the next 5 to 10 years. With medical and dietary therapy, however, the
recurrence rate can be significantly reduced.
Prevention strategies generally aim to correct under-lying risk factors with diet or medication. Dietary changes that can lower the
risk of calcium stones include an increase in fluid intake (sufficient to produce
a urine volume of >2 L daily), limitation of salt intake (which reduces urinary
calcium excretion), modest restriction of animal protein, and a reduction in
consumption of oxalate-rich foods (such as nuts, chocolate, brewed tea, and
dark green leafy vegetables). Severe dietary calcium restriction is discouraged
in any stone former, but for patients with elevated urinary calcium levels, a
modest reduction in calcium intake is recommended.
Medications can also help prevent stone formation. For
patients with calcium stones, thiazide diuretics can help reduce urinary
calcium excretion. For patients with uric acid stones, alkalizing agents such
as potassium citrate can increase uric acid solubility. In the minority of
patients with elevated urine uric acid levels, allopurinol may also be used.
Finally, underlying medical disorders that favor stone
formation should also be treated, such as hypercalcemia associated with
hyperparathyroidism.
BLADDER STONES
Primary bladder stones form within the bladder and are
distinct from stones that originate in the kidney and pass into the bladder.
Although bladder stones were common in the past, improvements in nutrition have
substantially reduced their incidence, since dietary phosphate deficiency and
excess ammonia excretion can contribute to stone formation. In developing
countries, however, bladder stones remain common.
In industrialized nations, bladder stone formation is
usually related to urinary stasis or urinary infection with urea-splitting
bacteria (e.g., Proteus mirabilis). Indeed, these conditions often
coexist, since urinary stasis predisposes to infection. Bladder stones are
typically com- posed of calcium phosphate, uric acid, or struvite.
The most common disorder associated with incomplete
bladder emptying and bladder stone formation is benign prostatic hyperplasia
(BPH). In affected patients, treatment consists of transurethral prostate
resection and laser or pneumatic stone fragmentation. In the case of a very
large prostate, open prostatectomy and bladder stone removal may be necessary.
Another disorder associated with bladder stone
formation is neurogenic bladder (see Plate 8-2), which occurs when neurologic
disorders such as spinal cord injury, multiple sclerosis, or spina bifida
interfere with normal voiding. Patients with neurogenic bladder who have long-term
indwelling catheters are particularly prone to bladder calculi because of their
increased rate of
infection with urea-splitting organisms. The bladder stones are most commonly treated with endoscopic fragmentation
and removal, with open surgery only rarely performed. The risk of further stone
formation can be decreased with intermittent rather than indwelling
catheterization, increased hydration, and bladder irrigation with weakly acidic
solutions, such as acetic acid. Antibiotics are rarely indicated because
bacteriuria is essentially unavoidable, and overuse of anti- biotics may
promote resistance.
The symptoms of bladder calculi are typically less
obvious than those associated with kidney stones. Some patients may be
completely unaware that they have a stone, while others may complain of urgency
and frequency of urination, pelvic pain, or hematuria. These symptoms are also
commonly associated with the underlying condition that leads to stone
formation, such as bladder outlet obstruction or bladder infection.






