T‐LYMPHOCYTES AND ANTIGEN‐PRESENTING CELLS INTERACT THROUGH SEVERAL PAIRS OF ACCESSORY MOLECULES
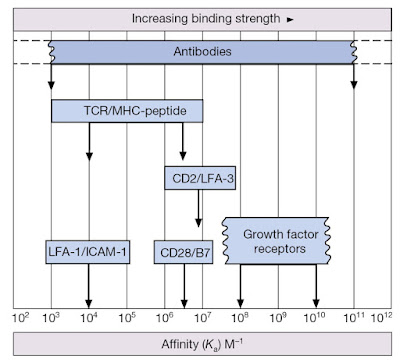
Before we delve into the nuts and bolts of TCR‐driven signaling events, it is important to recall that T‐cells can only recognize antigen when presented within the peptide‐binding groove of major histocompatibility complex (MHC) molecules. Furthermore, while the TCR is the primary means by which T‐cells interact with the MHC–peptide complex, T‐cells also express co‐receptors for MHC (either CD4 or CD8) that define functional T‐cell subsets. Recall that CD4 molecules act as co‐receptors for MHC class II and are found on T‐helper cell populations that provide “help” for activation and maturation of B‐cells and cytotoxic T‐cells (Figure 7.1). CD8 molecules act as co‐receptors for MHC class I molecules and are a feature of cytotoxic T‐cells that can kill virally infected or precancerous cells (Figure 7.1). Note, however, that the affinity of an individual TCR for its specific MHC–antigen peptide complex is relatively low (Figure 7.2). Thus, a sufficiently stable association with an antigen‐presenting cell (APC) can only be achieved by the interaction of several complementary pairs of accessory molecules such as LFA‐1/ICAM‐1, CD2/LFA‐3, and so on (Figure 7.3). These adhesion molecules enable T‐cells to associate with DCs and other APCs for the purposes of inspecting the peptides being presented within MHC molecules (Figure 7.4). However, these molecular couplings are not necessarily concerned with intercellular adhesion alone; some of these interactions also provide the necessary co‐stimulation that is essential for proper lymphocyte activation.
 |
Figure 7.1 Helper and cytotoxic T‐cell subsets are restricted by MHC class. CD4 on helper T‐cells acts as a co‐receptor for MHC class II and helps to stabilize the interaction
between the TCR and peptide–MHC complex; CD8 on cytotoxic T‐cells performs a similar function by associating with MHC class I.
 |
Figure 7.4 Interaction between T‐cells and dendritic cells. Scanning electron
microscopy analysis of DC–T‐cell
interactions within a 3‐D collagen matrix. |
Unstimulated lymphocytes are typically
nonadherent but rapidly
adhere to extracellular matrix components or other cells (such as APCs) within
seconds of encountering chemokines or antigen. Integrins such as LFA‐1 and VLA‐4 appear to be particularly important for lymphocyte
adhesion. The ease with which lymphocytes can alter their adhesiveness seems to
be related to the ability of integrins to change conformation; from a closed,
low‐affinity state to a more
open, high‐affinity one (Figure 7.5).
Thus, upon encounter of a T‐cell with an APC displaying an appropriate
MHC–peptide complex, signals routed
through the TCR complex ensure that the affinity of LFA‐1 for ICAM‐1 is rapidly increased and this helps to stabilize
the interaction between the T‐cell and the APC. This complex has come to be known
as the immunological synapse. Activation of the small GTPase Rap1
by TCR stimulation appears to contribute to the rapid change in
integrin adhesiveness. How Rap1 achieves this remains somewhat uncertain, but
it is likely that modification of the integrin cytoplasmic tail serves to
trigger a conformational change within the integrin extracellular domains in a
process that has been termed “inside‐out” signaling.
 |
Figure 7.5 Integrin activation. Integrins such as LFA‐1 can assume different conformations that are associated with different affinities. The bent head‐piece conformation has a low affinity for ligand but can be rapidly transformed into the extended high‐affinity conformation by activation signals that act on the cytoplasmic tails of the integrin α and β subunits; a process known as “inside‐out” signaling.