RENAL CELL CARCINOMA
Renal cell carcinomas (RCC) account for a vast majority of primary malignant renal tumors. Approximately 55,000 new cases are diagnosed in the United States each year, and about one third of patients have meta- static disease. Other, less common malignant renal tumors include transitional cell carcinomas of the renal pelvis (see Plate 9-9) and primary renal sarcomas. The kidneys may also contain metastases from extrarenal solid and hematologic tumors.
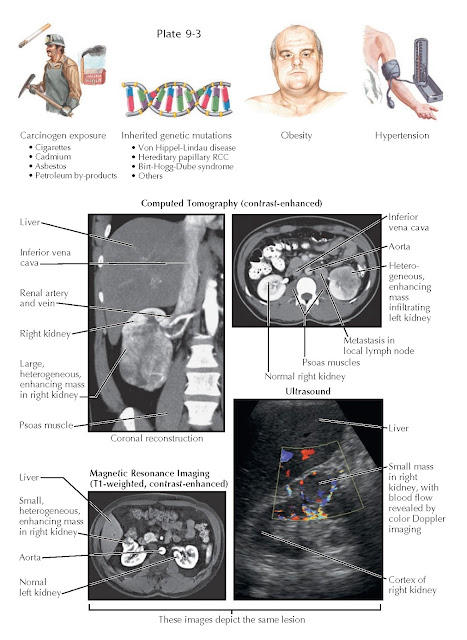
RISK FACTORS AND RADIOGRAPHIC FINDINGS OF RENAL CELL CARCINOMA
EPIDEMIOLOGY
AND RISK FACTORS
RCC was once
more than twice as common among men than among women, although this gap
currently appears to be shrinking. The peak incidence is in the sixth to
seventh decades of life. Environmental risk factors include cigarette smoking
and exposure to cadmium, asbestos, or petroleum byproducts. Data suggest that
cigarette smoking and cadmium exposure each double the risk, and that smoking
alone is responsible for one third of total cases. In addition, genetic
abnormalities in critical tumor suppressor genes and oncogenes are known to
play a key role. Such mutations can be sporadic or part of a hereditary condition,
such as von Hippel-Lindau disease, hereditary papillary RCC, and Birt-Hogg-Dube
syndrome. Hypertension and obesity also increase the risk for RCC, although the
mechanisms are not known. Although many tumors occur in patients without known
risk factors, it is likely that a significant number of “sporadic” cases will
eventually be found to have a genetic basis.
PRESENTATION,
DIAGNOSIS, AND STAGING
In the past,
an RCC was typically not detected until it became symptomatic, usually as the
classic triad of gross hematuria, flank pain, and a palpable mass. In
contemporary practice, however, the classic triad is seen in fewer than 10% of
patients. Instead, the majority of renal masses are now incidentally detected
during abdominal imaging.
Nonetheless,
an RCC may also cause a variety of nonspecific symptoms, including weight loss,
fever, night sweats, and lymphadenopathy. Some patients may also have dyspnea,
cough, and bone pain, which are suggestive of metastatic disease. Finally, RCC
can also be associated with a wide variety of paraneoplastic phenomena,
including erythrocytosis, anemia, hypercalcemia, hypertension, and
nonmetastatic hepatic dysfunction (Stauffer syndrome). Patients with any
combination of these symptoms or syndromes require immediate evaluation for
possible RCC.
The
evaluation of a known or suspected RCC begins with a thorough history and physical examination, with careful attention to the
symptoms listed previously. On laboratory assessment, possible abnormalities
include abnormal hematocrit, elevated erythrocyte sedimentation rate, elevated
serum calcium concentration, and abnormal liver function tests. Finally, a
careful evaluation of kidney function is important because it may have a
significant impact on the type of management if RCC is diagnosed. A normal serum
creatinine concentration is an acceptable assessment of renal function in
patients with no comorbidities
and normal-appearing kidneys on standard axial imaging. In patients with
medical conditions that predispose to renal disease, such as hyper-tension and
diabetes mellitus, assessing the function of each kidney with a nuclear scan
may be helpful for deciding between radical and nephron-sparing approaches.
Several
imaging techniques may be used to evaluate a suspected RCC, including
ultrasound (US), computed tomography (CT),
magnetic resonance imaging (MRI), renal angiography, and radionuclide imaging
(renography).
CT scan is
the most frequently employed modality because it has a high sensitivity for
detecting renal masses and is the most accurate. Any renal mass that enhances
with contrast is potentially malignant. CT also provides excellent
visualization of the adjacent structures into which the primary tumor can
extend or metastasize—such as the renal vein, regional lymph nodes, inferior
vena cava, and suprarenal (adrenal) glands—which enables accurate staging.
Ultrasound
can help identify the presence of a renal mass. Although ultrasound evaluation
does not require ionizing radiation and is less expensive than CT, it is less
sensitive and is highly dependent on operator skill. MRI is as sensitive as
CT and is the study of choice for patients who cannot receive iodinated
contrast.
The most
common sites for metastasis of renal cell carcinoma include the local and
thoracic lymph nodes, lungs, liver, bone, brain, ipsilateral suprarenal gland,
and contralateral kidney. Thus metastatic evaluation should include a chest
radiograph/CT scan and liver function tests. Bone scans may be indicated if the
patient complains of musculoskeletal pain, or if the serum calcium or alkaline
phosphatase concentrations are elevated.
Renal tumors
are generally not biopsied because of concerns regarding complications, the
false-negative result rates, and the fact that an overwhelming majority (90%)
of renal masses greater than 4 cm in diameter are malignant. In contemporary
practice, with more small renal tumors (4 cm) being identified, the role of
renal biopsy is actively being reevaluated, and it is likely that in the future
renal biopsy will become a more common practice. Potential complications
include bleeding, infection, needle track seeding, and pneumothorax. In
addition, false negatives for malignancy do occur.

GROSS PATHOLOGIC FINDINGS IN RENAL CELL CARCINOMA
TREATMENT
The optimal
treatment of RCC depends largely on the tumor stage, size, and location, as
well as the patient’s overall clinical condition.
Localized
disease can be surgically treated with radical resection (see Plate 10-19),
nephron-sparing surgery (such as partial nephrectomy [see Plate 10-22] or
ablation [see Plate 10-24]), or observation with an active surveillance
protocol. In contrast, unresectable or metastatic RCC in patients with good
functional status is
commonly managed with initial cytoreductive surgery followed by systemic
medications, such as interleukin 2 and tyrosine kinase inhibitors. In patients
with poor functional status, medical therapy alone is used.
Localized
Disease. Radical nephrectomy was previously the initial standard of care for the
treatment of all localized RCCs. The operation involves complete removal of the
kidney and suprarenal (adrenal) gland within the renal fascia, as well as
removal of regional lymph nodes from the crus of the diaphragm to the aortic
bifurcation. The surgery can be performed using either an open or laparoscopic approach and results in
an extremely low local recurrence rate (2% to 3%). Laparoscopic radical
nephrectomy, however, has become increasingly popular in recent years because
of shorter recovery times and equivalent oncologic outcomes when compared with
the open approach. Thus
it is now considered the treatment of choice for patients with localized tumors
less than 10 cm in diameter with no local invasion, renal vein involvement, or lymph node metastasis.
Partial nephrectomy is an alternative to
radical resection that preserves renal function in the affected kidney. The
procedure can be performed using either an open or laparoscopic approach. In recent years, partial nephrectomy has
become the standard of care for patients with tumors that are fewer than 4 cm
in diameter. This option can be especially important in patients with decreased
renal function, a solitary kidney, or a chronic disease that may affect
long-term renal function. Careful preoperative and intraoperative imaging is
required to adequately identify the tumor’s borders and its relationship to
major intrarenal vessels and the collecting system.
Ablative
procedures, including cryosurgery and radiofrequency ablation, are newer
nephron-sparing techniques that have been studied as alternatives to partial
nephrectomy. These techniques can be per- formed from either a percutaneous or
laparoscopic approach, and they are associated with shorter recovery times and
decreased morbidity. Successful treatment requires adequate intraoperative
imaging to ensure optimal placement of the ablation probes, as well as
repetitive ablative cycles to ensure complete tumor destruction. Although these
procedures are safe and well-tolerated, long-term oncologic data are still
relatively limited. The preliminary data, however, demonstrate that recurrence
rates may be slightly higher than those following traditional surgery.
Nonetheless, ablative techniques are useful options for many patients,
including those with contraindications to conventional surgery, those with
multiple lesions (in whom partial nephrectomy would be difficult), or those with
recurrent disease that requires focal salvage therapy.
In patients
who are elderly or are poor candidates for surgery for other reasons,
observation is a reasonable alternative. It is generally reserved for patients
with small (3 cm) renal lesions. There is no established standardized protocol
for active surveillance; however, most clinicians perform serial imaging every
6 to 12 months to assess for disease progression.
 |
| HISTOPATHOLOGIC FINDINGS IN RENAL CELL CARCINOMA |
Metastatic
Disease. In the past, patients with advanced RCC were not viewed as candidates
for surgical resection, given their poor prognosis. Recently, however,
advancements in adjuvant therapies have changed the role of surgery in the
management of metastatic disease. In patients with good performance status and
limited metastatic disease, the goal of surgical resection is to completely
remove all affected tissue, including nearby organs and/or abdominal wall muscles. In addition,
careful removal of solitary metastases has been shown to improve 5-year
survival rates in some patients. Such interventions are cytoreductive and have
been shown to improve outcomes if performed before the initiation of adjuvant
therapy.
Several
biologic agents have been recently developed and studied as treatment for
disseminated clear cell RCC. Tyrosine kinase inhibitors, such as sorafenib and sunitinib, have been
found to inhibit tumor growth and angiogenesis by blocking the vascular
endothelial growth factor receptor (VEGF-R). Meanwhile, bevacizumab is an
monoclonal antibody that has also been shown to be effective, and which acts by
directly binding circulating VEGF. Additional agents with proven efficacy
include mTOR (mammalian target of rapamycin) inhibitors, such as temsirolimus
and everolimus. Finally, high-dose interleukin 2 (IL-2) can activate an immune response against the tumor, with modest response rates. The effect of
these various agents on the growth and overall prognosis of non–clear cell
tumors is unclear and remains under active investigation.
PATHOLOGY/GRADING
There are
several variants of RCC, which are distinguished based on histomorphology. In
addition, there are cytogenetic abnormalities that correlate with the
histologic findings. Renal cell carcinoma variants include clear cell (75% to
85%, arising from the proximal tubule), papillary (15%, also arising from the
proximal tubule, sometimes termed chromophil), chromophobe (5%, arising from
intercalated cells of the cortical collecting duct), unclassified (5%),
multilocular clear cell (rare), renal medullary (rare), Xp11 translocation
(rare), mucinous tubular, spindle cell (rare), and collecting duct (rare). The
histologic features of the most common tumor types are shown in Plate 9-5.
For clear
cell carcinomas, the Fuhrman nuclear grading system has prognostic significance
and should always be used; it grades these tumors from 1 to 4 based on nucleus
size, nucleus shape, and nucleolus appearance. The use of this grading system
in non–clear cell carcinomas is less well established.
PROGNOSIS
The
prognosis of treated RCC depends on numerous variables, including tumor stage,
histopathologic findings, presence or absence of symptoms, laboratory values,
and the patient’s overall performance status. The tumor stage is the most
significant factor, since the 5-year survival of a TNM stage I tumor has been
found to be approximately 95%, whereas that of a stage IV tumor is less than
25%. Several scoring systems have been devised to calculate the overall
prognosis based on various factors.

STAGING SYSTEM AND SITES OF METASTASIS IN RENAL CELL CARCINOMA
FOLLOW-UP
Although
there is no standard recommendation for follow-up of patients who have
undergone surgical resection of localized RCC, the frequency and intensity of
the protocol are generally dictated by the clinical tumor stage,
histopathology, and treatment strategy. Patients are at greatest risk for recurrence in the first 5 years.
Many centers
determine their follow-up schedule based on tumor stage. Patients with
localized tumors that are less than 7 cm in diameter (T1) are at lowest risk
for recurrence. Such patients should undergo annual evaluation that consists of
a physical examination, chest radiograph, and laboratory testing of liver and
kidney function. Some experts recommend measurement of serum alkaline
phosphatase concentrations to monitor for bone metastases; however, the sensitivity and specificity
of this laboratory marker are poor.
Patients
with masses that are larger than 7 cm or extend into adjacent structures (T2-4)
are at higher risk for recurrence and, in addition to the above, should also
undergo annual CT scan. Finally, all patients who have undergone a
nephron-sparing procedure require an additional CT scan 3 months after the
procedure to evaluate the tumor resection site for local disease recurrence.




