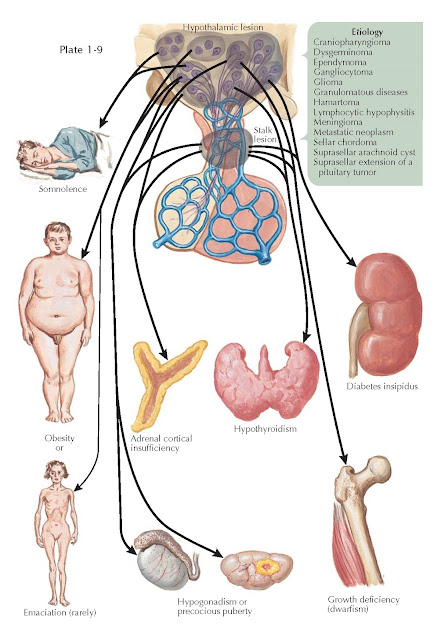
MANIFESTATIONS OF SUPRASELLAR DISEASE
Suprasellar lesions that may lead to hypothalamic dysfunction include craniopharyngioma, dysgerminoma, granulomatous diseases (e.g., sarcoidosis, tuberculosis, Langerhans cell histiocytosis), lymphocytic hypophysitis, metastatic neoplasm, suprasellar extension of a pituitary tumor, glioma (e.g., hypothalamic, third ventricle, optic nerve), sellar chordoma, meningioma, hamartoma, gangliocytoma, suprasellar arachnoid cyst, and ependymoma.
Endocrine and nonendocrine sequelae
are related to hypothalamic mass lesions. Because of the proximity to the optic
chiasm, hypothalamic lesions are frequently associated with vision loss. An
enlarging hypothalamic mass may also cause headaches and recurrent emesis. The
hypothalamus is responsible for many homeostatic functions such as appetite
control, the sleep–wake cycle, water metabolism, temperature regulation,
anterior pituitary function, circadian rhythms, and inputs to the
parasympathetic and sympathetic nervous systems. The clinical presentation is
more dependent on the location within the hypothalamus than on the pathologic
process. Mass lesions may affect only one or all of the four regions of the
hypothalamus (from anterior to posterior: preoptic, supraoptic, tuberal, and
mammary regions) or one or all of the three zones (from midline to lateral:
periventricular, medial, and lateral zones). For example, hypersomnolence is a
symptom associated with damage to the posterior hypothalamus (mammary region)
where the rostral portion of the ascending reticular activating system is
located. Patients with lesions in the anterior (preoptic) hypothalamus may
present with hyperactivity and insomnia, alterations in the sleep–wake cycle
(e.g., nighttime hyperactivity and daytime sleepiness), or dysthermia (acute
hyperthermia or chronic hypothermia).
The appetite center is located in
the ventromedial hypothalamus, and the satiety center is localized to the
medial hypothalamus. Destructive lesions involving the more centrally located
satiety center lead to hyperphagia and obesity, a relatively common
presentation for patients with a hypothalamic mass. Destructive lesions of both
of the more laterally located feeding centers may lead to hypophagia, weight
loss, and cachexia.
Destruction of the vasopressin-producing
magnocellular neurons in the supraoptic and paraventricular nuclei in the
tuberal region of the hypothalamus results in central diabetes insipidus (DI)
(see Plate 1-27). In addition, DI may be caused by lesions (e.g., high
pituitary stalk lesions) that interrupt the transport of vasopressin through
the magnocellular axons that terminate in the pituitary stalk and posterior
pituitary. Polydipsia and hypodipsia are associated with damage to central
osmoreceptors located in anterior medial and anterior lateral preoptic regions.
The impaired thirst mechanism results in dehydration and hypernatremia.
Anterior pituitary function control
emanates primarily from the arcuate nucleus in the tuberal region of the
hypothalamus. Thus, lesions that involve the floor of the third ventricle and
median eminence frequently result in varying degrees of anterior pituitary
dysfunction (e.g., secondary hypothyroidism, secondary adrenal insufficiency, secondary hypogonadism, and growth
hormone deficiency).
Hypothalamic hamartomas,
gangliocytomas, and germ cell tumors may produce peptides normally secreted by
the hypothalamus. Thus, patients may present with endocrine hyperfunction
syndromes such as precocious puberty with gonadotropin-releasing hormone
expression by hamartomas; acromegaly or Cushing syndrome with growth
hormone–releasing hormone expression or
corticotropin-releasing hormone expression, respectively, by hypothalamic
gangliocytomas; and precocious puberty with -human chorionic gonadotropin
(-hCG) expression by suprasellar germ cell tumors.
Because of the close microanatomic
continuity of the hypothalamic regions and zones, patients with suprasellar
disease typically present with not ne but many of the
dysfunction syndromes discussed.