INITIAL FORMATION
OF THE BRAIN AND SPINAL CORD: THE EMBRYO AT 20 TO 24 DAYS
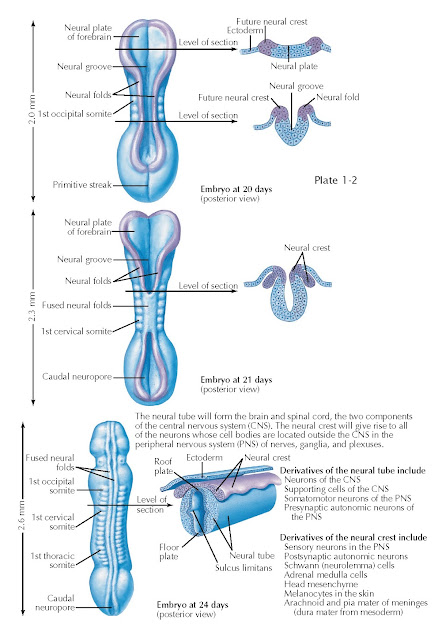
As gastrulation ends, another series of cell movements—neurulation—transforms the neural plate into a tube of neural stem cells: the neural tube. Neurulation is accompanied by elaboration of mesodermal tissues into somites that form the axial skeleton and musculature, and visceral differentiation by the endoderm. These events cooperate to yield an embryonic nervous system that consists of a tube surrounding a fluid-filled cavity that will eventually form the brain ventricular system (see below) and the spinal cord central canal. The geometry of the neural plate and underlying notochord remains the primary determinant of how the neural tube forms. By day 20 of development, the neural plate has thickened and flexed upward from the midline, right above the notochord. The neural crest, initially specified at the lateral (or alar) margins of the neural plate is relocated to the posterior midline as the rest of the neural plate forms a tube by joining the neural folds at each lateral margin of the neural plate at the posterior midline.
By embryonic day 21, the neural tube
in the midsection of the embryo has closed; the neural folds fuse and the
underlying neuroectoderm encloses a fluid-filled cavity that becomes the spinal
cord central canal. The neural crest delaminates at the posterior midline. This
epithelial (cell sheets) to mesenchymal (loosely arrayed, motile cells between
the sheets) transition of neural crest cells is much like the epithelial to
mesenchymal transition that occurs in many cancers of mature epithelial
tissues. For the neural crest, however, this transition begins a highly
regulated process of migration to multiple peripheral locations where neural
crest precursors continue to divide and differentiate into sensory ganglia
(cranial and posterior root), autonomic ganglia, enteric neurons, pigment
cells, components of the posterior aorta, cranial bones, and connective
tissues. The neural plate at the anterior and posterior ends of the embryo has
begun to fold into a neural tube but has not yet reached the point where
lateral margins meet and fuse at the posterior midline. The midline “hinge
point” where neural tube formation begins is visible as the neural groove anteriorly
and the rhomboid sinus posteriorly, and the forebrain neural plate as
well as the posterior spinal cord remain open to the extraembryonic
environment.
Within another 3 days, by embryonic
day 24, the neural tube is closed from the anterior end
(where the brain will form) throughout much of the length of the spinal cord,
with the exception of an opening at the rhomboid sinus or posterior neuropore.
At this stage, the neural tube has begun to acquire additional signs of
differentiation that reflect the genesis of neurons with distinct functions.
First, based upon the location of either the notochord, or the alar region and
neural crest, two regions of the neural tube become specialized to provide
signals to the rest of the neuroepithelial neural stem cells that constitute
the developing nervous system. The neural tube cells above the notochord at the
anterior midline constitute the floor plate, and those at the fusion of
the neural folds at the posterior midline become the roof plate. Floor
plate and roof plate cells secrete signals that influence neighboring cells in
the neural tube, such as sonic hedgehog, a peptide hormone that regulates
proliferation and differentiation. These signals further distinguish the
presumptive spinal cord and hindbrain into anterior/basal and posterior/alar regions (see also Plate 1-6), separated by a
midline groove referred to as the sulcus limitans (this structure is not
always easy to see).
These geometrically defined domains
of neural stem cells generate functionally distinct classes of neurons. The
neural stem cells of the posterior/alar region will generate sensory projection
and interneurons that relay and process incoming sensory information from
peripheral sensory ganglia, and those in the anterior/basal region will give
rise to motor neurons that project to peripheral muscles and autonomic ganglia,
as well as interneurons that modulate the output of motor neurons. Signals from
the roof plate and floor plate elicit local expression of transcription factors
and other determinants in neighboring neural stem cells. These factors define
the capacity of the local stem cells to generate distinct classes of sensory
and motor projection or interneurons.