CARDIAC STRESS IMAGING
Stress imaging studies combine either EST or an infusion of either dobutamine or a coronary vasodilator with imaging of the heart. Imaging can be accomplished by a variety of modalities; those most commonly used are echocardiography or nuclear imaging. MRI has also been used, and CT is being studied as a modality for stress imaging. Stress imaging is preferred over EST without imaging in several settings: (1) when the ECG is uninterpretable for myocardial ischemia; (2) when a patient is unable to adequately exercise (but can undergo a pharmacological stress imaging study); or (3) when a treadmill stress test is positive for ischemia in a low-risk patient, and correlation by imaging is preferred to cardiac catheterization. Individuals with an abnormal baseline ECG, particularly with ST-segment abnormalities, should be referred for a stress imaging study, because ECG changes in the setting of an abnormal baseline are far less specific for CAD. Patients with significant left ventricular hypertrophy on their baseline ECG or those taking digoxin have similar limitations for interpretation of ischemia with exercise. Stress imaging could be used as a primary modality, rather than ECG-only stress testing, in patients with an intermediate to high pretest likelihood of disease because of its higher sensitivity and specificity. Even with rapid advances in other modalities, stress imaging remains a highly effective and available modality to evaluate ischemia and function at present, and it is likely that this will be the case in coming years.
Myocardial Perfusion Imaging
Myocardial perfusion imaging (MPI) involves injection of a radiopharmaceutical
that distributes throughout the myocardium in a manner dependent upon coronary
blood flow. Images are obtained of the radio- pharmaceutical distribution
attained near peak stress and at rest. Changes in the distribution of the
radiopharmaceutical can reflect comparable blood flow at rest and during
stress, diminished blood flow with stress compared with rest (reflecting
stress-induced ischemia), or diminished blood flow both with stress and at
rest, which can be correlated with previous myocardial infarction (MI). Left
ventricular function and ejection fraction (EF) and left ventricular size at
rest and with stress can also be measured with this technique. The sensitivity
of stress nuclear imaging for detection of hemodynamically significant CAD is 85%
to 90%. The prognostic value of a negative stress nuclear imaging study is also
excellent in otherwise low-risk to intermediate-risk patients.
Imaging can be done with SPECT or with PET. These systems offer different
spatial resolution and use different tracers; however, the basic approach of
stress perfusion and the functional images obtained are essentially the same.
Stress With Myocardial Perfusion Imaging
In stress with MPI, the radiopharmaceutical is injected when the patient is at the maximum level of coronary
vasodilation, which occurs at peak exercise
or pharmacological stress. Exercise stress is preferred for MPI because of the added prognostic
information obtained from the hemodynamic response to exercise and functional
tolerance. Exercise improves imaging characteristics of the tracers, leading to
fewer artifacts and improved accuracy.
The same previously noted contraindications noted for EST apply to
patients undergoing exercise MPI. Many of the limitations inherent in ECG-only
exercise testing (e.g., left bundle branch block, pacing, atrial fibrillation,
left ventricular hypertrophy, and baseline ST- and T-wave changes) can largely
be overcome when using MPI. In general, the sensitivity and specificity of MPI
for detection of CAD are better when coupled with exercise than when coupled
with pharmacological stress. For this reason, if a patient is able to exercise,
exercise MPI is preferred.
When patients are unable to exercise (due to poor functional capacity,
orthopedic, or other factors) or have a significant left bundle branch block,
MPI can be performed using pharmacological stress. Two general approaches are
used in pharmacological stress testing: infusion with a coronary vasodilator or
with dobutamine.
Dipyridamole, adenosine, and regadenoson are coronary vasodilators used
in pharmacological stress MPI. Dipyridamole causes vasodilation by blocking
endogenous adenosine breakdown and raising its levels. Adenosine can also be
directly infused and is preferred in many centers over dipyridamole because it
results in a more consistent serum adenosine level (and more consistent
coronary vasodilatation) than does the infusion of dipyridamole. Adenosine
infusion is associated with more symptoms than dipyridamole infusion, but these
symptoms are short- lived because of its short half-life. Regadenoson acts more
specifically on the coronary adenosine (A2α) receptors compared with
the nonspecific vasodilation action of pure adenosine. Thus, it should have a
lower risk of common side effects (e.g., bronchospasm, atrioventricular nodal
blockade, and flushing), although these side effects can still be present.
Coronary vasodilators work by increasing blood flow except in areas where
hemodynamically significant stenoses are present, precluding vasodilator-induced
increased flow. A relative decrease in the intensity of the MPI signal
indicates an inability to increase flow to that area of the myocardium, and
therefore, the presence of flow-limiting CAD in the coronary artery supplying that area can be deduced.
The use of vasodilators is contraindicated in patients with active
bronchospastic disease, and in those with advanced heart block or sick sinus
syndrome without a pacemaker. In addition, patients taking aminophylline or
theophylline must discontinue the use of these drugs before vasodilator
pharmacological stress testing, because these drugs counteract the effects of
these vasodilators. Similarly, caffeine intake within the previous 12 hours
also blocks the effects of vasodilators. Regadenoson is associated with a lower
seizure threshold and is often avoided in patients with a history of seizures.
Some also avoid its use in end-stage renal disease. If a patient receiving
vasodilators does have either bronchospasm or another side effect with drug
infusion, these side effects can be mitigated by infusion of aminophylline or
theophylline. It is rare that reversal of the effects of adenosine is required
because of its short half-life.
If patients are able to perform submaximal exercise, a combination of a vasodilator
with exercise can be performed. This protocol has the advantages of decreasing
side effects and improving image quality by decreasing splanchnic tracer
accumulation. Vasodilator-exercise protocols allow limited exercising of
patients who are not able to attain target HRs. However, patients with
contraindications to either exercise or vasodilators (see the preceding text)
should not be considered for a combined stress study. In addition, vasodilator
EST should not be performed in patients with a history of cerebrovascular
and/or carotid disease, especially if walking is the exercise mode. Rapid loss of consciousness and collapse on the treadmill have been reported, due to cerebrovascular perfusion steal, which
results from pharmacological vasodilation coupled with exercise (Fig. 10.3).
 |
| FIG 10.3 Pharmacological Stress Nuclear Testing |
If patients are unable to exercise and also have contraindications to vasodilator stress, dobutamine pharmacological stress can be performed. Dobutamine is more often used for stress echocardiography than for stress MPI, and is similar to exercise in that it increases HR and myocardial contraction. It is administered as an incrementally increasing infusion rate until either the MPHR of the patient or the infusion rate is reached. Atropine can be used for HR augmentation if the target HR is still not reached with maximal dobutamine doses. Stress targets are similar to those for exercise, but it is important to note that because systolic blood pressure can remain constant or fall with dobutamine, whereas it rises with exercise, the double product (and thus, level of stress) associated with a given HR is less during dobutamine testing than with exercise testing. Some clinical variables, such as fatigue, which is useful in EST, are generally not useful with dobutamine administration. The major contraindications to dobutamine and/or atropine stress MPI are the same as for EST, but also include the presence of narrow- angle glaucoma, and a history of prostatic enlargement and urinary obstruction. In addition, a relative contraindication to dobutamine and/or atropine stress MPI is a propensity for inducible arrhythmias.
Finally, less conventional stress methods such as cold pressor testing
and mental stress are described in the literature. Mental stress testing is
believed to induce a sympathoadrenal response that has similar effects on the
coronary blood flow as the previously described methods, which induces the
steal phenomenon and exposes perfusion defects. However, the exact mechanism is
unknown. The mental stress test coupled with echocardiography may improve
sensitivity of the test but not the specificity. Low sensitivity of the test
can be due to inability to identify the stressors that may induce ischemia in a
patient. Cold pressor testing is more often used when coronary vasospastic
syndromes are suspected, and reduce blood flow to areas where such vasomotor
dysfunction exists, thereby inducing ischemia.
An imaging protocol for acute chest pain involves administration of a
radiopharmaceutical while the patient is having a chest pain syndrome. In a
low-risk to intermediate-risk patient, a normal scan has a high negative
predictive value for the absence of an acute coronary syndrome. This protocol
has been used in emergency room settings in low-risk to intermediate-risk
patients with otherwise undifferentiated chest pain and allows for safe
discharge with outpatient follow-up.
Thallium-201 (201Tl) thallous chloride, a radioactive analogue
of potassium, was the most commonly used tracer for myocardial perfusion for
several decades. Although its use has declined with the advent of
technetium-99m (99mTc)–based agents, it is still sometimes used as
part of dual-isotope protocols and in viability imaging. Its relatively low
energy results in images that lack resolution, although it has a higher
myocardial extraction fraction compared with 99mTc-based agents. The
two most commonly used 99mTc-based MPI agents are 99mTc-sestamibi
(MIBI) and 99mTc-tetrofosmin. Images obtained with the two agents
are comparable and have a higher resolution than images obtained using 201Tl
for cardiac imaging. MIBI demonstrates a slightly higher extraction fraction
than tetrofosmin, although it results in a slightly higher radiation dose to
the patient compared with tetrofosmin. A previously used 99mTc-based
agent, teboroxime, demonstrated a substantially higher extraction fraction than
the aforementioned agents, but its rapid washout from the myocardium limited
its clinical usefulness. Teboroxime is no longer marketed in the United States.
The 99mTc-agents are the most commonly used SPECT
radiopharmaceuticals. Several imaging protocols using these agents have been developed. A commonly used protocol is the
1-day rest-stress, wherein a scan is
performed following a low dosage tracer administration to the patient at rest.
The second step in this protocol is to stress the patient (exercise or
pharmacological stress), administering the resting dosage of the radiotracer at
peak stress approximately three times, and then perform imaging again.
A variation of this protocol used in some nuclear laboratories for
low-risk patients is the stress-rest study. In this case, stress images are
obtained first. Resting images can be omitted if the stress images are completely
normal, but can be performed on the same or subsequent day if needed. In the
former, a lower dosage stress image is obtained followed by a higher dosage
rest image. The disadvantage of this approach is that stress images are
obtained at lower doses of radiotracer and thus may be of lower quality. A
2-day protocol administration of relatively high dosages of radiopharmaceutical
is used for both rest and stress. This protocol allows for better image
quality, especially in obese patients in whom high-quality images cannot
otherwise be attained. The limitations of this study protocol are the higher
radiation doses and the inconvenience of having the patient return on a
subsequent day.
A dual-isotope protocol uses 201Tl for the resting images
followed by poststress images obtained with a 99mTc-based tracer.
However, differences in spatial resolution between 201Tl and 99mTc
can sometimes complicate the interpretation of subtle findings. Imaging can
also be performed using 201Tl only. Because of the limitations of 201Tl,
the only feasible approach is to perform a stress-rest study. The entire study
can be performed with a single injection of tracer, and one can obtain
additional physiological and prognostic information (e.g., lung uptake) and an
assessment of myocardial viability. However, these studies are not done
frequently in most laboratories because they are associated with significantly
higher radiation doses than those only using 99mTc- agents, are more
time-consuming, and provide lower resolution images. PET radiopharmaceuticals
use positron-emitting radionuclides to create images. Rubidium-82 (82Rb)
chloride is a positron-emitting potassium analogue. It has the lowest
extraction fraction of the clinically
available PET radiopharmaceuticals (~60%). This extraction fraction is still
higher than that of either sestamibi or tetrofosmin. The half-life of 82Rb
is short (~75 seconds). There are benefits and limitations for the use of 82Rb
because of its short half-life. The short half-life essentially precludes use
of 82Rb for exercise stress imaging. However, it facilitates
obtaining images when the patient is truly at the peak of performance induced
by pharmacological stress. For this reason, 82Rb images can be used
to accurately assess cardiac reserve, which are defined as the difference
between left ventricular EF at rest and at peak stress. The short half-life of 82Rb
also facilitates obtaining pharmacological stress and resting images in a
relatively short period of time. 82Rb has a lower intrinsic spatial
resolution than the other PET agents but is still far better than the SPECT
tracers. 82Rb is produced by a generator system, but this is quite
expensive; for this reason, it is only available at some centers.
Nitrogen-13 (13N) ammonia has a high extraction fraction
(~83%), higher imaging resolution than 82Rb, and a 10-minute
half-life. It can be used for exercise nuclear imaging. Oxygen-15 ([15O]H2O)
water is short-lived (half-life of 2 minutes) and possesses a high extraction
fraction of approximately 95%. However, its freely diffusible nature means that
15O is distributed into tissues adjacent to the myocardium,
including the lungs and cardiac blood pool. For this reason, imaging is
complicated, requiring sophisticated background subtraction techniques. Although
both 13N and 15O have higher intrinsic spatial resolution
than 82Rb, they require generation in a cyclotron. Their short half-lives mean
that these isotopes can only be used in facilities with an on-site cyclotron.
For most institutions that perform PET myocardial imaging studies, 82Rb is
preferred for this logistic reason. Newer fluorine-18 (18F)-labeled perfusion tracers that would allow exercise imaging and do not
require an on-site cyclotron are
being developed and studied. The 18F tracers have a high extraction
fraction and the highest imaging resolution, making them attractive for the
assessment of CAD.
PET tracers use protocols based on SPECT imaging. Because of its
exceedingly short half-life, 82Rb protocols can be either
rest-stress (more common) or stress-rest. An entire 82Rb study can
be completed within 30 minutes. An advantage of PET tracers is that despite
higher γ-emission energies, their radiation doses are comparably lower while
delivering better images than the SPECT tracers.
There is increasing interest in stress first protocols in both SPECT and
PET modalities. This allows significant radiation and costs reduction, as well
as shortens the overall procedure time for the patient. The approach is more
feasible with newer technologies, such as iterative image processing,
attenuation correction, and cardiac-specific cameras because they can help
eliminate many imaging artifacts. These technologies are not specifically
reimbursed, but may significantly increase the cost of the procedure to the
laboratory, which is a barrier to wider adoption. Nevertheless, the imaging
community has dedicated itself to overall radiation dose reduction in an era of
rising concerns about radiation exposures from diagnostic testing and
healthcare cost containment.
With the variety of techniques available, it is important to choose the
optimal imaging modality (SPECT vs. SPECT-CT vs. PET), tracer, stress modality,
and imaging protocol, tailoring each for the specific patient situation to
maximize the information obtained. For example, the overall prognosis of a
normal stress MPI study is better in patients who exercised than in those who
were evaluated with a vasodilator study. Careful attention should be paid to
understanding the meaning of the results in the context of the history of the
patient and how the study was performed. The study design also has to be
weighed against the technological abilities of the stress laboratory,
throughput, procedure costs, and the
radiation exposure of the patient.
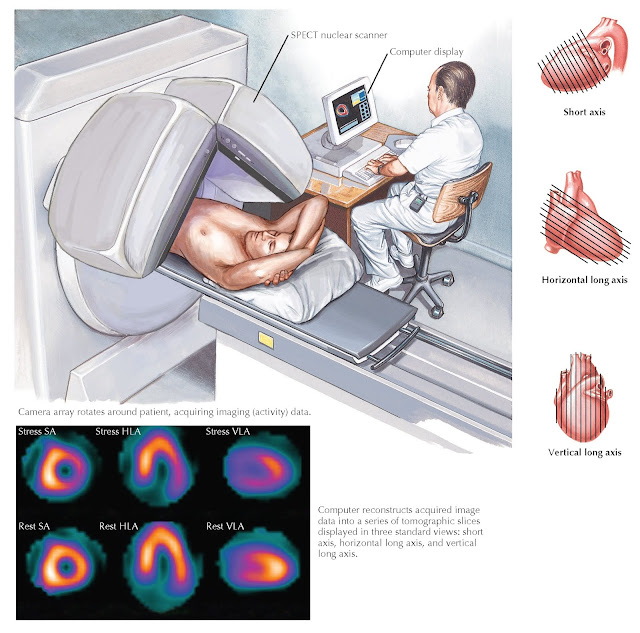 |
FIG 10.4 Stress Nuclear Imaging by SPECT. Lateral wall shows normal perfusion at rest, but
decreased
|
at
stress consistent with ischemia. |
IMAGE INTERPRETATION
SPECT nuclear images are analyzed in three ways. The “raw” rotating image interpretation is a critical step
that allows the reader to assess whether patient motion, attenuation artifacts
(breast overlap, diaphragmatic interference, or other factors) must be
considered in interpretation of the study. Occasionally, the presence of
significant extracardiac findings such as breast or lung masses, thyroid or
parathyroid nodules, and lymphadenopathy is seen on these raw images. The
second step is to examine reconstructed images that are presented as “slices”
of the myocardium. Using this set of images, it is possible to visualize
myocardial perfusion in multiple axes to assess flow-limiting CAD (Fig. 10.4
and Table 10.1). The amount of ischemic or infarct
burden can be quantified. By dividing the ventricle into segments (usually 17
or 20) and then deriving scores based
on the extent and severity of segments affected by pathology, a quantitative assessment can be made that strongly
correlates with patient outcomes. The summation of these data, the “sum score,”
can be compared in the rest and stress studies. Third, gated images can also be
obtained and reviewed in a looped-cine method. These images allow determination
of wall motion abnormalities, ventricular volumes, and left ventricular EFs.
Analysis of wall motion also provides an independent means to assess apparent
perfusion defects and confirm infarction, ischemia, or the presence of an
artifactual perfusion abnormality.
Comparison of images obtained at stress with images obtained at rest makes it possible to determine if there is a relative decrease in flow with stress. This reversible myocardial perfusion defect correlates with viable tissue in the distribution of a coronary artery with a significant stenosis and represents an ischemic territory. If a portion of the myocardium has limited perfusion at stress and at rest, this indicates that the myocardium with the nonreversible defect may represent an infarcted area.
Due to the higher isotope energies involved with PET imaging, its
inherent attenuation correction, and the superior tracer characteristics of PET
radiopharmaceuticals compared with the current 99mTc-based SPECT
agents, PET images are of far superior quality and usefulness in the diagnosis
of CAD in both obese and general patients. The approach to interpretation of
PET imaging is similar to that described previously for SPECT imaging. Reconstructed perfusion and gated images are approached the same way, but no raw images
are displayed because of the manner in which PET images are acquired. An
important step to consider in PET is the alignment of the emission and
transmission (the latter being CT in PET-CT or MR in PET-MR units) scans. By
default, PET has an attenuation correction built in for the reconstruction of
its final images. A misalignment between the two portions of the scan can
result in serious artifacts, which can be misread if not recognized and/ or
corrected. Although this can be frequently corrected by manual realignment of
the images, occasionally, the relevant scan has to be repeated to obtain the
correct data.
PET imaging also makes absolute quantification of myocardial blood flow
and coronary flow reserve possible, which is useful for detection of
endothelial dysfunction, and assessment of multivessel ischemia that might
otherwise appear as normal stress imaging if ischemia is global and balanced.
ADVANCES AND INTEGRATION OF CARDIAC
IMAGING
Numerous important innovations in cardiac nuclear imaging have improved the diagnostic performance of the
field. More sophisticated processing using iterative reconstructive techniques
has allowed for imaging and technological developments in the field of nuclear
imaging, specifically for cardiac imaging. Some solutions involve using nuclear
SPECT cameras and developing “cardiocentric” collimators. Other solutions have
developed SPECT cameras specifically for cardiac imaging, where the patient
sits upright, thus improving patient comfort. Other cardiac-specific cameras
use multi-pinhole designs and are without moving parts. Newer cameras use cadmium
zinc telluride high-efficiency, solid-state detectors, but these significantly
increase the costs of these systems.
It is useful to consider nuclear imaging techniques (SPECT and PET) with
newer cardiac imaging technologies such as cardiac MRI (CMRI) and cardiac CT
angiography (CTA), because their use has increased dramatically within the last
few years; there are advantages and disadvantages for each. CMRI is capable of
generating exquisite images of cardiac structures, with a resolution far superior
to nuclear techniques and without the need for ionizing radiation. This
technology is also useful for viability assessment and nonischemic
cardiomyopathies. The current major limitation to the use of CMRI in stress
testing is its limited availability.
Cardiac CTA provides high-resolution images of coronary and other cardiac
anatomy and pathology that are not possible with current nuclear techniques,
with radiation doses somewhat comparable to SPECT imaging but higher than those
of PET. Although its negative predictive value for the detection of CAD is
excellent, its positive predictive value in determining disease severity is
considerably lower. It is anticipated that as technology advances, cardiac CTA
characterization of coronary anatomy will improve.
It is also possible that CT technology will be able to provide a combined
scan that includes stress testing, viability assessment, and coronary anatomy,
all in a reasonable time frame and with an acceptable radiation dose. However,
there are limitations of these newer technologies. For patients with renal
insufficiency who have a higher risk of allergic or nephropathic complications,
nuclear tracers are preferred over studies that require intravenous contrast
(either CT or MRI). MRI studies are generally contraindicated in patients with
implanted cardiac rhythm devices (pacemakers and ICDs; coronary stents are not
a contraindication for MRI). At present, CMRI and cardiac CTA are less widely
available than nuclear studies.
Ultimately, the combination of imaging modalities may provide the greatest noninvasive information for
cardiac patients. Combined modality imaging has proven to be useful for
detection and prognostication in cancer patients. The idea of combining
high-resolution images with physiological and/or functional measures is equally
attractive for the assessment of CAD. The integration of CT into both SPECT and
PET imaging devices can be useful for anatomic localization of perfusion
defects and for attenuation correction, particularly in obese patients, and is
important for PET perfusion studies.
Furthermore, the perfusion and/or metabolic information provided by SPECT
or PET can be obtained sequentially, and then fused with structural information
provided by CTA. This approach offers the potential advantage of evaluating
both the extent and severity of atherosclerotic vascular disease and its effect
on myocardial perfusion, and can also be of great use in distinguishing between
flow-limiting coronary artery stenosis and microvascular disease.
Similarly, PET and MRI have been combined as a PET-MR device that also
overcomes the major limitations of poor anatomic resolution in MPI, while
overcoming the limited spatial coverage in stress MRI. Development of new
tracers could expand the clinical potential of PET-MR imaging, which would
allow highly specific assessment of vascular atherosclerosis, nonischemic
cardiomyopathies, and HF. All these advancements have allowed the reduction of
imaging time and tracer dosages, which leads to a decrease in overall patient
radiation exposures.





