BENIGN PROSTATIC HYPERPLASIA II: SITES OF HYPERPLASIA
AND ETIOLOGY
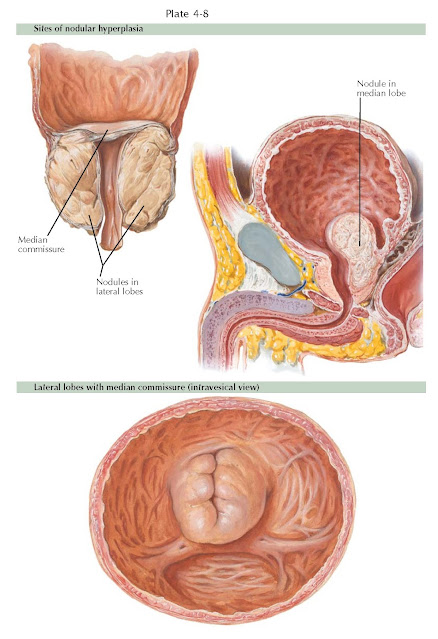
Prostatic enlargement due to benign prostatic hyperplasia (BPH) presents with several typical growth configurations. The nodules usually enlarge in a symmetrical manner, although in some instances one side may predominate. On rectal examination, this may present as prostatic asymmetry and, without induration or nodularity, is not suspicious for carcinoma. As nodules grow, they have been termed median, lateral, and anterior lobe hyperplasia, according to their location cystoscopically. These designations are misnomers because the prostate is generally not a lobulated organ. These terms simply designate general cystoscopic locations.
The most frequent types of prostatic enlargement are “bilobular” (the two
lateral lobes) and “trilobular” (the two lateral plus the median lobe)
hyperplasia. Isolated median lobe enlargement may occur without lateral lobe
enlargement, but it is much less common. Rarely, nodules can originate in the
roof of the urethra within the anterior zone and project downward into the
bladder, giving the appearance of a rounded “anterior” lobe.
With lateral lobe hyperplasia, the nodules grow to surround the prostatic
urethra; as a result, it becomes considerably elongated. Growth is confined
within the prostate without projection into the bladder neck. The lateral lobes
may grow to great size, with only a minimal degree of urinary obstruction. When
they extend into the bladder neck, this projection may interfere with the
opening of the bladder neck and result in urinary obstruction. Median lobe
enlargement begins in the posterior urethra and, following the line of least
resistance, projects as a mass up through the bladder neck and into the
bladder. Other nodular enlargement occurs in the vicinity of the Albarrán
glands (see Plate 4-2) just beneath the bladder neck and tends to produce
intravesical hypertrophy.
The degree of BPH cannot be accurately judged from the rectal
examination, which only reveals the size of lateral lobe enlargement below the
bladder neck. As such, the rectal examination does not accurately correlate
with the degree of obstruction or with voiding symptoms.
The origin of BPH remains unclear. Because hyperplasia arises mainly
within the transition zone that contains an unusual juxtaposition of glandular
and stromal elements, one theory purports that BPH develops as a consequence of
stromal cell dysregulation and is induced by the effects of androgens and
estrogens. This change in the prostatic stromal–epithelial cell interactome
that occurs with age may lead to an inductive effect on prostatic growth and is
termed the embryonic reawakening theory. Another theory, termed the DHT
hypothesis, suggests that a shift in prostatic androgen metabolism occurs with aging that leads to an abnormal accumulation of dihydrotestosterone
(DHT) and stimulating prostatic enlargement. The stem cell theory of BPH
advocates an increase in the total prostatic stem cell number and/or an
increase in the clonal expansion of stem cells into amplifying and transit
cells with age. What is clear is that aging and the presence of a functional
testis are the two established risk factors for the development of BPH. There
are ethnic trends in occurrence, as the condition
is more common in Caucasian and black men than it is in Asian men. Vascular
disease, infection, and sexual activity do not correlate with BPH, but it is
less likely to occur in hypogonadal men. The active androgen responsible for
prostatic growth and development is DHT, and the administration of 5-alpha
reductase inhibitors, commonly prescribed for urinary symptoms due to BPH, will
reduce prostatic volume by one-third over 6 months.