THE EVOLUTION OF METABOLISM
Because cells originated in a sea of organic molecules, they were able to obtain food and energy directly from their environment. But such a situation is self-limiting, so cells needed to evolve their own mechanisms for generating energy and synthesizing the molecules necessary for their replication. The generation and controlled utilization of metabolic energy is central to all cell activities, and the principal pathways of energy metabolism (discussed in detail in Chapter 3) are highly conserved in present-day cells. All cells use adenosine 5′-triphosphate (ATP) as their source of metabolic energy to drive the synthesis of cell constituents and carry out other energy-requiring activities, such as movement (e.g., muscle contraction). The mechanisms used by cells for the generation of ATP are thought to have evolved in three stages, corresponding to the evolution of glycolysis, photosynthesis, and oxidative metabolism (Figure 1.4). The development of these metabolic pathways changed Earth’s atmosphere, thereby altering the course of further evolution. In the initially anaerobic atmosphere of Earth, the first energy-generating reactions presumably involved the breakdown of organic molecules in the absence of oxygen. These reactions are likely to have been a form of presentday glycolysis the anaerobic breakdown of glucose to lactic acid, with the net energy gain of two molecules of ATP. In addition to using ATP as their source of intracellular chemical energy, all present-day cells carry out glycolysis, consistent with the notion that these reactions arose very early in evolution. Glycolysis provided a mechanism by which the energy in preformed organic molecules (e.g., glucose) could be converted to ATP, which could then be used as a source of energy to drive other metabolic reactions. The development of photosynthesis is generally thought to have been the next major evolutionary step, which allowed the cell to harness energy from sunlight and provided independence from the utilization of preformed organic molecules. The first photosynthetic bacteria probably utilized H2S to convert CO2 molecules a pathway of photosynthesis still used by some bacteria. The use of H2O as a donor of electrons and hydrogen for the conversion of CO2 to organic compounds evolved later and had the important consequence of changing Earth’s atmosphere. The use of H2O in photosynthetic reactions produces the by-product free O2; this mechanism is thought to have been responsible for making O2 abundant in Earth’s atmosphere, which occurred about 2.4 billion years ago.
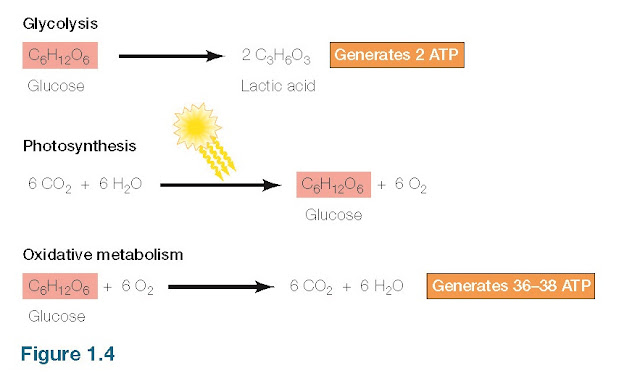 |
Generation of metabolic energy Glycolysis
is the anaerobic break- down of glucose to lactic acid. Photosynthesis utilizes energy from sunlight to drive the
synthesis of glucose from CO2 and H2O, with
the release of O2 as a
by-product. The O2 released
by photosynthesis is used in oxidative metabolism, in which glucose is broken
down to CO2 and H2O, releasing much more energy
than can be obtained glycolysis.
The release of O2 as a consequence of photosynthesis changed the environment in which cells evolved and is commonly thought to have led to the development of oxidative metabolism. Alternatively, oxidative metabolism may have evolved before photosynthesis, with the increase in atmospheric O2 then providing a strong selective advantage for organisms capable of using O2 in energy-producing reactions. In either case, O2 is a highly reactive molecule, and oxidative metabolism, utilizing this reactivity, has provided a mechanism for generating energy from organic molecules that is much more efficient than anaerobic glycolysis. For example, the complete oxidative breakdown of glucose to CO2 and H2O yields energy equivalent to that of 36 to 38 molecules of ATP, in contrast to the 2 ATP molecules formed by anaerobic glycolysis (see Figure 1.4). With few exceptions, present-day cells use oxidative reactions as their principal source of energy.




