OCHRONOSIS
Ochronosis is the name
given to the later clinical findings of alkaptonuria. Alkaptonuria is caused by
an inborn error of metabolism resulting from a defect or deficiency of the
enzyme homogentisic acid oxidase. A complete lack of the enzyme in the kidneys
and liver is responsible for the buildup of the homogentisic acid. Alkaptonuria
is transmitted in an autosomal recessive manner. Homogentisic acid oxidase is
responsible for the metabolism of homogentisic acid, which is a break- down
product of the amino acids phenylalanine and tyrosine. This enzyme metabolizes
homogentisic acid into maleylaceto-acetic acid, which is eventually con- verted
to fumaric acid and aceto-acetic acid. When, as in alkaptonuria, the
homogentisic acid oxidase enzyme is deficient, homogentisic acid accumulates in
the blood and is excreted in the urine. The disease has a slow, insidious
onset, and patients often present initially in young adulthood.
Clinical Findings: The first clinical sign is that of dark urine found in an affected
baby’s diaper, which often causes concerned parents to seek medical advice. If
left to stand for a few minutes, the urine turns dark black because of the
oxidative effects of the atmosphere. The urine can be alkalinized with a strong
basic solution such as sodium hydroxide; addition of the basic solution to a sample of urine promptly turns it dark
black. Benedict’s reagent can also be used to test the urine of patients with
alkaptonuria; when it is added, the supernatant turns dark black, and this
finding is diagnostic of alkaptonuria.
As the homogentisic acid accumulates in these
patients, it eventually begins depositing in skin and cartilage tissue, for
which it has an affinity, becoming visibly noticeable in the fourth decade of life. The sclera is one of
the first areas to be noticeably involved. A subtle brown discoloration begin
to form on the lateral aspect of the sclera and continues to darken over the
lifetime of the patient. The ear cartilage becomes dark brown to almost bluish
due to the accumulation of the homogentisic acid. The cerumen is dark black,
and evaluation of the ear may also show a darkening of the tympanic membrane and the stapes, incus, and malleus
bones of the inner ear. The patient may suffer from tinnitus.
With time, the skin in various regions begins to
become hyperpigmented. This occurs first and foremost in the areas with a high
concentration of sweat glands. The axillae and the groin are noticeably
affected. The excessive homogentisic acid is secreted in the sweat, and the
pigment discolors the surrounding skin. The cheeks are also prominently affected.
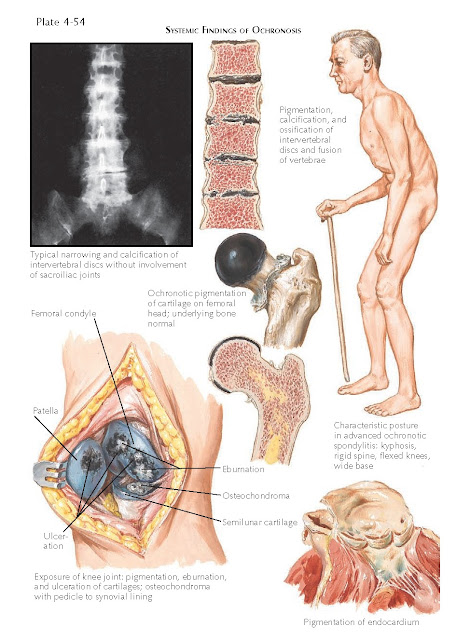
The most disabling aspect of this disease is the
deposition of homogentisic acid in the fibrocartilage and hyaline cartilage.
This leads to severe degenerative joint disease at an early age. The pigment
alters the cartilage and makes it brittle and friable. The cartilage begins to
fragment and disintegrate and can get embedded in the synovial tissue, causing
synovial polyps. The intervertebral disks become severely pigmented and begin
to calcify because of the massive destruction of the cartilage. The disks are
destroyed, causing a severe reduction in the patient’s height, as well as
chronic pain and rigidity of the spine. Eventually, the heart, prostate, aorta,
and kidneys all show evidence of ochronosis.
Pathogenesis: Ochronosis is
the result of an autosomal recessive inherited disorder that causes the
affected patient to be deficient in the enzyme homogentisic acid oxidase. This
deficiency, over time, leads to the accumulation of homogentisic acid in various tissues throughout
the body and the subsequent clinical manifestations.
Histology: The findings
on skin biopsy are pathognomonic for ochronosis. Large ochre bodies are found
within the dermis. These are obvious on low-power microscopy and can be used to
confirm the diagnosis.
Treatment: No known cure
is available, and there is no effective therapy. Physical therapy and joint replacement increase flexibility and range of motion
and help to decrease morbidity. Some researchers advocate a diet low in
phenylalanine and tyrosine, although the success of this approach is anecdotal
at best. The National Institutes of Health is currently studying an inhibitor
of the enzyme 4-hydroxyphenylpyruvic acid dioxygenase, which would decrease the
production of homogentisic acid and theoretically help to decrease joint destruction.