Adrenal Insufficiency
Primary adrenal insufficiency
Primary adrenal failure, or Addison’s disease, arises as a result
of a destructive process in the adrenal gland or genetic defects in steroid
synthesis. All three zones of the adrenal cortex are typically affected.
Symptoms and signs
The onset is usually gradual. Symptoms may be non-specific, hence
it is important to maintain a high index of suspicion for the diagnosis. Most
commonly, patients describe fatigue, weakness, anorexia, weight loss, nausea
and abdominal pain. Dizziness and postural hypotension occur as a result of
mineralocorticoid deficiency whereas glucocorticoid loss leads to
hypoglycaemia, and increased pigmentation as a result of ACTH excess (leading
to melanocyte stimulation) from reduced cortisol negative feedback. Androgen
deficiency in women can lead to reduced libido and loss of axillary and pubic
hair.
Causes
There are several causes of primary adrenal failure but autoimmune
adrenalitis is by far the most common cause in Western populations, and is
supported by detection of positive adrenal autoantibodies. Other causes are
rare but should be considered when antibody testing is negative (Table 20.1).
Investigations
Routine laboratory tests show hyponatraemia (>90%),
hyperkalaemia, raised urea, hypoglycaemia and a mild anaemia. However, specific
tests are needed to make the diagnosis. A low 09.00 cortisol and simultaneously
raised ACTH concentration is suggestive of the diagnosis, although a Synacthen
test is generally needed for confirmation (Table 20.2).
Management
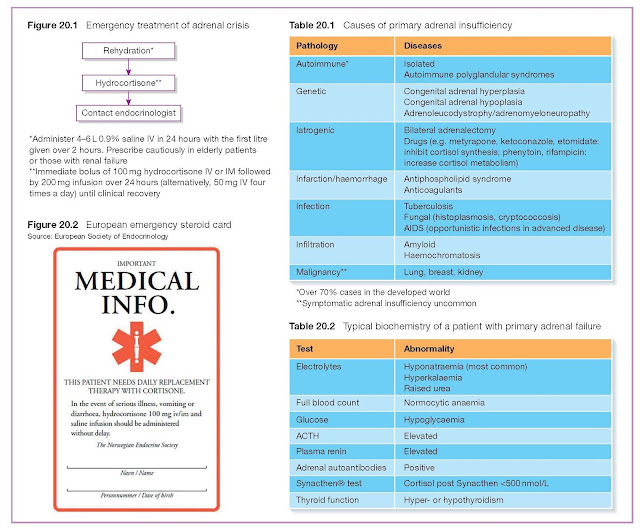
Emergency treatment of adrenal crisis
This is considered in Figure 20.1 and also in Chapter 35.
Maintenance treatment
Patients with primary adrenal failure need lifelong glucocorticoid
and mineralocorticoid replacement therapy. Hydrocortisone is the glucocorticoid
of choice, which is given in total daily doses of 15–30 mg, divided into
two (e.g. 10 mg twice daily) or three doses (e.g. 10 mg on waking,
5 mg at lunchtime and 5 mg in the early evening). Mineralocorticoid
replacement is given as fludrocortisone 50–200 µg once daily.
Patients should be instructed to double the dose of their
glucocorticoid at times of illness, and continue on a doubled dose until their
illness has resolved. Glucocorticoids need to be administered IV or IM during
surgery or in cases of prolonged vomiting or diarrhoea. Patients should be
provided with a steroid emergency card (Figure 20.2), encouraged to wear
medical alert jewellery and be provided with emergency contact details for their
endocrine team.
Secondary adrenal insufficiency
Secondary hypoadrenalism can arise as a result of any cause of
hypopituitarism (Chapter 5). Patients display similar symptoms and signs to
primary adrenal insufficiency, with the exception that pigmentation is absent,
as ACTH is not raised, and mineralocorticoid deficiency is not a feature,
because aldosterone secretion is not significantly influenced by ACTH. As with
primary adrenal failure, diagnosis relies upon a failure to demonstrate a rise
in cortisol following Synacthen administration, coupled with demonstration of
an inappropriately low/low–normal plasma ACTH level. The insulin stress test
can also be used to diagnose ACTH deficiency (Chapter 2). The principles of
hydrocortisone replacement and dose adjustment are the same as for primary
adrenal failure but fludrocortisone replacement is not required as
mineralocorticoid secretion is intact.
Steroid-induced hypoadrenalism
Corticosteroids are frequently prescribed as anti-inflammatory
drugs. An important consequence is suppression of the HPA axis, particularly
when prescribed in high doses and/or over a long period of time. Consequently,
sudden cessation of long-term therapy can lead to adrenal crisis. Patients
taking long-term steroids should thus be instructed not to stop their steroids
abruptly, at least until an adequate adrenal reserve has been demonstrated. As
with other causes of adrenal insufficiency, patients should carry a steroid
card and be educated about steroid supplementation at times of illness.