Achalasia and
Diffuse Esophageal Spasm
Achalasia is an uncommon disease occurring in 1:10,000 to 1:100,000 people. It
results from neurodegeneration of the myenteric plexuses in the esophageal body
and lower esophageal sphincter, leading to esophageal aperistalsis and
incomplete opening of the lower esophageal sphincter. Whereas the former
results from a lack of excitatory input to stimulate peristalsis, the latter
occurs from decreased nitric oxide mediated inhibitory input, leaving the
sphincter in a baseline excitatory state with failure to relax in response to
deglutition.
The initiating event in the pathophysiology remains unclear, but
both genetic predisposition and postviral autoimmune mechanisms have been
described. Clinically, it is a slow progressive process often masquerading as
reflux and typically with a long delay in diagnosis. Without effective
treatment, the process continues with progressive dilation of the esophagus, at
times, to massive pro portions with compression of adjacent structures such as
the lung and trachea. Classic symptoms of achalasia are dysphagia to liquids
and solids, regurgitation, chest pain, and weight loss. Achalasia patients,
however, often learn to adjust their lifestyle to the disease and present with
more subtle accommodating symptoms such as slow eating and stereotactic
movements with eating, such as sitting up straight or walking during a meal.
These maneuvers physiologically increase the longitudinal muscle tone. The
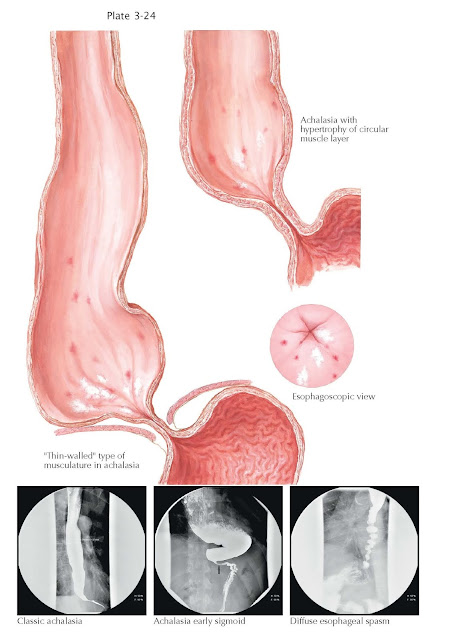
diagnosis is made by a combination of compatible symptoms, imaging (radiography
and/or endoscopy), and esophageal manometry. Imaging demonstrates a range of
findings depending on the severity of the disease. In early stages, a
nondilated esophagus with a difficult to pass or incompletely opening lower
esophageal sphincter may be seen on endoscopy or radiography. As the disease advances,
esophageal dilation is more easily appreciated, often with retained saliva and
food present despite prolonged fasting. In the most advanced stages, the
esophagus may elongate and dilate similar in appearance to the colon in a
process described as “sigmoidization.” Without the ability to alter the
underlying neural injury, therapy is aimed at pharmacologic or mechanical
disruption of the lower esophageal sphincter to at least allow gravity to
facilitate passage of the bolus into the stomach. A simple but relatively
shortterm treatment is endoscopic injection of botulinum toxin into the lower
esophageal sphincter. Pharmacologically, this suppresses cholinergic
stimulatory activity and lowers the lower esophageal sphincter pressure.
Mechanical therapies include endoscopy dilation with a highpressure pneumatic
balloon to rip sphincter muscle fibers or more precise cutting of the sphincter
(myotomy) through a surgical approach. Recently, the latter has been performed
completely through endoscopy (peroral endoscopic myotomy) by tunneling through
the esophageal submucosa and then incising the inner circular layer of the
muscularis propria of the lower esophageal sphincter. Longterm results are not
available yet for this novel approach. In severely advanced achalasia with
sigmoidization, esophagectomy may be needed. Finally, there is an increased
risk of esophageal squamous cell cancer in patients with longstanding
endstage disease. The pathophysiology of diffuse esophageal spasm is
likely similar to that of achalasia. The pathophysiology, however,
reflects a more pronounced form of esophageal disinhibition. Specifically, it
is manifested by an incompletely relaxing lower esophageal sphincter but also a
shorter time interval from the onset of deglutition to lower esophageal
sphincter relaxation (decreased distal latency) and hypertensive esophageal
contractions. These patients have dysphagia and/or severe episodic chest pain.
Radiographically, a “corkscrew” esophagus may be seen. Treatment is similar to that
for achalasia, but the response rate of symptoms, particularly chest pain, is
not as robust when compared with the response of dysphagia in achalasia. As a
result, additional treatment to control the chest pain is often needed. The
treatments are aimed at decreasing esophageal muscle pressures (e.g., with
anticholinergic or nitric oxide enhancing medications) or reducing esophageal
sensation (e.g., with lowdose tricyclic antidepressants). The number of cases
of diffuse esophageal spasm that remain stable or progress
to more typical forms of achalasia is variable.