RENAL ARTERY
STENOSIS
Renal artery stenosis (RAS) is an uncommon but important cause of
secondary hypertension. It is unclear what fraction of hypertension is related
to this problem; however, it is currently estimated that 1% to 2% of patients
with mild to moderate hypertension have clinically significant RAS. Establishing
whether the RAS is the primary cause of hypertension in such patients is
difficult.
In addition to its effects on blood
pressure, RAS can also lead to impaired renal function, a phenomenon known as ischemic
nephropathy.
PATHOPHYSIOLOGY
A substantial portion of the aging
population has some degree of RAS, which may be discovered as an incidental
finding during color Doppler ultrasound or other vascular imaging studies.
Indeed, roughly 20% to 45% of the patients who undergo angiography for any
indication will be found to have RAS. Once the stenosis occludes more than
approximately 50% to 70% of the arterial lumen, a significant drop in pressure
distal to the lesion produces a series of pathophysiologic events that lead to
a fall in renal blood flow and rise in systemic arterial pressure.
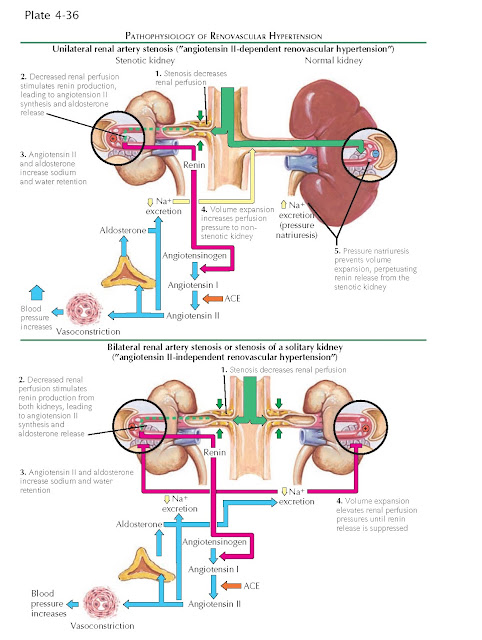
First, reduced perfusion pressure
to the affected kidney decreases hydrostatic pressure in the glomeruli and
thereby reduces tubular flow rates, triggering release of renin and synthesis of
angiotensin II and aldosterone. These hormones increase systemic pressure and
promote volume retention, leading to secondary hypertension.
If the contralateral kidney is
normal, it will be exposed to these circulating hormones and initially
contribute to volume expansion. As its perfusion pressure increases above
normal, however, the contralateral kidney will begin to excrete sodium and
water. This phenomenon, known as “pressure natriuresis,” relies on mechanisms
that are incompletely understood. Although autoregulation generally prevents
increased perfusion pressure from reaching the glomerular capillaries, it has
been hypothesized that increased shear stress in the preglomerular vessels, as
well as increased renal interstitial hydrostatic pressure, may activate local
natriuretic mechanisms. As a result, the nonstenotic kidney prevents effective
volume expansion, and the persistently underperfused stenotic kidney continues
to secrete renin. At least in the early stages, the hypertension is thus
angiotensin-dependent; however, later in the disease course, renin levels fall
as alternate pressor mechanisms, such as endothelin and oxidative stress, are
recruited.
If, in contrast, the contralateral
kidney is absent or dysfunctional, or if both kidneys are affected by RAS,
renin secretion will lead to unopposed expansion of fluid volume. Once there is
enough volume to achieve normal perfusion pressure in the stenotic kidney (or
kidneys), renin secretion ceases.
During these processes, the
affected kidney may itself become dysfunctional, a phenomenon known as
“ischemic nephropathy.” The kidney as a whole does not become “ischemic” per se
because its blood supply generally continues to exceed overall metabolic requirements.
Nonetheless, the decline in pressure causes autoregulation to become
ineffective, leading to focal areas of tissue injury and ischemia. In addition,
the hemodynamic changes lead to altered expression of endothelium-derived
substances, such as nitric oxide and endothelin, and promoters of fibrogenic injury,
such as transforming growth factor β. As a result, the kidney may exhibit a
variable degree of tubulointerstitial fibrosis. If there is bilateral disease,
overall filtration may become impaired.
The RAS itself reflects the presence
of either atherosclerotic disease, which accounts for approximately 90% of
cases, or fibromuscular dysplasia, which accounts for most of the remainder.
Atherosclerosis is a common
problem, especially among individuals over 50 years of age. It is associated
with risk factors including smoking, diabetes mellitus, and
hypercholesterolemia. Because hypertension is also a well-known risk factor for
atherosclerosis, many patients with atherosclerotic RAS may also have essential
hypertension. Atherosclerosis typically affects the proximal
region of the renal artery and the perirenal aorta.
Fibromuscular dysplasia comprises a
group of angiopathies that typically occur in women of child-bearing age. Their
etiology is unknown. These disorders can lead to fibroplasia in all layers of
the arterial wall, but most cases involve the media. A smaller number of cases
may feature intimal hyperplasia, which typically leads to dissection and
eventual thrombosis. Unlike atherosclerosis, fibromuscular dysplasia typically
affects the distal two thirds of the renal arteries.
PRESENTATION AND DIAGNOSIS
Few, if any, clinical features can
distinguish patents with renovascular hypertension from those with essential hypertension. Although some
features are suggestive, none is particularly sensitive or specific.
In the clinical history, suggestive
features include the onset of hypertension before age 30 or after age 50 (which
favor fibromuscular dysplasia or atherosclerosis, respectively); an acute rise
in blood pressure in patients with previously well-controlled essential
hypertension; refractory hypertension despite multiple treatments; accelerated
or malignant hypertension; and the presence of other vascular disease.
On physical examination, an
abdominal bruit may be noted. On laboratory studies, suggestive features
include hypokalemia (reflecting increased potassium secretion secondary to
aldosterone release), an increased BUN : creatinine ratio (reflecting increased
proximal tubule reabsorption secondary to angiotensin II release); a significant
elevation in serum creatinine concentration after starting an ACE inhibitor or
ARB; and a lack of evidence for intrinsic renal disease (e.g., benign urine
sediment). On abdominal imaging, one kidney may also appear markedly smaller
than the other in the setting of unilateral disease.
If the index of suspicion is high,
more specific tests may be performed, but these should be performed mainly if an
interventional procedure would be undertaken in the event that RAS were confirmed.
Noninvasive tests include
measurement of plasma renin (including after administration of captopril, which
removes the negative feedback from angiotensin II), as well as nuclear scanning
of renal function after captopril administration. These tests, however, are not
highly sensitive or specific in many populations because, for the reasons
discussed previously, renin secretion varies widely.
Imaging studies may be performed to
directly evaluate the renal vasculature, including Doppler ultrasound (US), computed
tomographic angiography (CTA), or magnetic resonance angiography (MRA). US may
reveal increased flow velocities across the narrowed vessel, and calculation of
the resistive index (which indicates small-vessel disease and parenchymal
fibrosis) may indicate the potential benefit of intervention. US, however, is
operator-dependent and varies widely between institutions.
CTA and MRA, in contrast, are
highly sensitive tests that are widely available; however, these sometimes fail
to detect lesions associated with fibromuscular dysplasia, which affect the more
distal segments of the renal artery. In addition, these tests require the use
of iodinated contrast or gadolinium, which limits their availability to
patients with reduced kidney function. Because of its risks and costs, invasive
angiography is generally not performed unless an intervention is planned for
the same procedure.
For patients with ambiguous degrees
of vascular occlusive disease, demonstrating lateralization of renal vein renin
levels reliably predicts the role of the affected kidney in sustaining
hypertension and the likely effect of revascularization on arterial pressure.
TREATMENT
ACE inhibitors or ARBs should be
offered to patients with RAS, either alone or in combination with other antihypertensives,
to lower systemic blood pressure. In unilateral disease, the nonstenotic kidney is
typically able to compensate for the reduced filtration that these agents cause
in the affected kidney. In bilateral disease, however, some patients will
experience a clinically significant decline in overall glomerular filtration rate
in response to these agents. Thus, in all patients, serum creatinine and
potassium concentrations should be measured shortly after these agents are
initiated.
In patients with atherosclerotic disease,
measures should be taken to limit the progression of plaque formation,
including smoking cessation and administration of statins.
The indications for renal
revascularization are controversial, particularly for patients with
satisfactory blood pressure control and stable kidney function. In general,
interventions should be considered in patients who have drug-resistant or
malignant hypertension. In addition, intervention should be considered in
patients who have either bilateral stenosis or stenosis to a solitary kidney
along with normal or mildly impaired renal function and no evidence of
intrinsic renal disease. Although it is difficult to predict which patients with
renal dysfunction will benefit the most from revascularization, some evidence
suggests that patients with high resistance indices on ultrasound are unlikely
to regain much function because they are more likely to have chronic,
irreversible renal disease.
Endovascular repair is generally
the preferred method of intervention. It consists of balloon angioplasty and,
in patients with atherosclerosis, stent placement. Surgical bypass of the renal
artery may be indicated in patients with complex lesions.