HIV-ASSOCIATED NEPHROPATHY
Several years after the
emergence of the acquired immunodeficiency syndrome (AIDS) epidemic in the early
1980s, an association with renal disease was recognized. By 1984, reports
described a distinctive form of focal segmental glomerulosclerosis (FSGS) in
African Americans and Haitian immigrants with AIDS living in the large urban
centers of New York and Miami. This new disease, initially called AIDS
nephropathy, is now termed human immunodeficiency virus (HIV)- associated
nephropathy (HIVAN) because the essential feature is infection with the HIV-1
virus, not the full clinical constellation of AIDS.
At present,
approximately 800 to 900 new cases of HIVAN are reported to the U.S. Renal
Database System (USRDS) each year. HIVAN is approximately tenfold more common
in blacks than whites, indicating a strong racial predisposition based on
genetic factors. It is clinically characterized by progressive renal
insufficiency, often accompanied by proteinuria, nephrotic syndrome, and the
ultrasonographic findings of enlarged, hyperechoic kidneys. The clinical picture
reflects virus mediated podocyte injury
and proliferation, which leads to collapsing focal segmental glomerulosclerosis
with microcystic dilation of the tubules and interstitial fibrosis and
inflammation.
In the early
years of the epidemic, before effective therapy, progression to end-stage renal
disease (ESRD) or death was nearly universal, and by 1999 “AIDS nephropathy”
had become the third leading cause of ESRD among adult African Americans aged
20 to 64 years. The widespread availability of combination antiretroviral
therapy to treat HIV-1 infection, however, has changed the natural history and
epidemiology of HIVAN. The incidence of new cases of HIVAN has been reduced and
the rate of progression to renal failure has been slowed by antiretroviral
therapy, which is now the mainstay of treatment.
Following the
introduction of combination antiretroviral therapy, the incidence of ESRD
attributed to HIVAN has reached a plateau in the United States; however,
because HIV-infected patients are living longer with nephropathy, the
prevalence of HIV-related ESRD continues to increase. Given a stable annual
mortality rate and assuming a linear growth of the HIV epidemic among African
Americans, it is projected that nearly 10,000 patients in the United States
will be living with ESRD due to HIVAN by the year 2020. Emerging data from
African populations indicate a high prevalence of kidney disease among
HIV-infected individuals in sub-Saharan Africa, reaching 38% in Nigeria. As
antiretroviral therapy becomes more available worldwide, it is likely that an
increasing number of HIV-infected Africans will also be living with ESRD due to
HIVAN.
PATHOPHYSIOLOGY
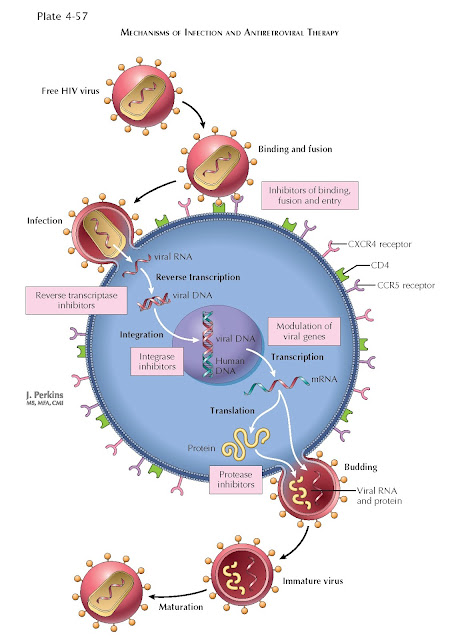
HIVAN is
caused by direct infection of renal epithelial cells by the HIV-1 virus,
leading to viral gene expression. RNA in situ hybridization and DNA in situ
polymerase chain reaction amplification of specific HIV-1 genes from human renal
biopsies have detected HIV-1 virus in the podocytes (glomerular visceral
epithelial cells), the parietal epithelial cells lining Bowman’s capsule, and
tubular epithelial cells. Individual patients are noted to have different HIV-1
quasispecies in their renal epithelium compared with their peripheral blood
leukocytes, indicating the ability of the virus to replicate and undergo
mutation within the renal epithelium.
This process of error-prone viral replication allows the virus to change its
coat and evade the host immune system. How the virus enters renal epithelial
cells is uncertain because there is no evidence of renal epithelial expression
of CD4 (the major HIV receptor in T helper cells) or the HIV-1 coreceptors,
CXCR4 and CCR5. It is possible that HIV-1 infects renal epithelium via
transcytosis from infiltrating lymphocytes.
Once the
HIV-1 virus enters renal epithelium, it expresses viral genes that can cause
cellular injury by inducing dysregulation of host genes. The HIV-1 genome contains a total
of nine genes, including genes that encode structural proteins (gag, pol,
env), regulatory proteins (tat and rev), and accessory
proteins (vif, vpr, vpu, nef). The use of genetically
engineered mice has identified several genes as particularly important in HIVAN
pathogenesis, namely nef (which augments viral replication and
infectivity) and vpr (which transports the HIV-1 preintegration complex
into the nucleus and induces cell cycle arrest). In the podocyte, expression of
nef activates signaling cascades that disrupt the actin cytoskeleton,
causing foot process effacement and failure
to maintain the normal filtration barrier. Heavy glomerular proteinuria and
nephrotic syndrome ensue. The infected podocytes revert to a more immature
phenotype resembling that seen in proliferating podocytes during glomerular
development. The inability of the podocyte to maintain its normal mature
phenotype leads to cellular dedifferentiation, proliferation, and glomerular
tuft collapse. The dysregulation of tubular epithelial cells by viral
infection, compounded by the tubular injury caused by severe proteinuria, leads
to tubular microcyst formation, interstitial fibrosis, and progressive renal
failure. Tubular expression of vpr causes G2 cell cycle arrest and
impairs cytokinesis of tubular epithelial cells, leading to increased
chromosomal copy number. As a result, infected tubular epithelial cells appear
hypertrophied with atypical enlarged nuclei.
Host factors
are also critical to disease pathogenesis. The vast majority of patients with HIVAN are of African descent.
Recently, a candidate gene has been linked to the development of HIVAN in this
group: APOL1, encoding apolipoprotein L-1, located on human chromosome
22. An APOL1 variant appears to have emerged in the African population
through a broad evolutionary sweep by conferring selective advantage against
infection by Trypanosoma brucei rhodesiense, a parasite that causes
sleeping sickness. APOL1 encodes a serum factor contained in high
density lipo- protein particles that lyses the trypanosomal organism. The
evolutionary selection of this genetic variant is analogous to the emergence of
hemoglobin mutations that confer protection against malaria at the price of
increased susceptibility to hemoglobinopathy and sickle cell anemia. In the
case of APOL1, protection against trypanosomal infection comes at the
cost of increased susceptibility to HIVAN and other forms of FSGS, although the
renal cellular mechanisms are unknown. Like the mutations underlying sickle
cell disease and trait, APOL1’s protective effect against infection is
dominant (present in heterozygotes), whereas the association with host disease
is recessive (occurring in homozygotes).
PRESENTATION
AND DIAGNOSIS
In the early
years of the AIDS epidemic, before anti-retroviral therapy, the classic
clinical presentation of HIVAN was rapidly progressive renal failure
accompanied by moderate to severe nephrotic-range proteinuria, bland urinary
sediment, and the ultrasound findings of large, highly echogenic kidneys.
Patients progressed to ESRD within several months.
Although
some cases have been reported in the setting of asymptomatic HIV infection or
acute HIV seroconversion, HIVAN is typically a complication of advanced HIV
disease. Thus HIV-infected patients who develop nephrotic-range proteinuria and
have a CD4 cell count less than 200 cells/mm3 should be strongly suspected of
having HIVAN. A renal biopsy is required to establish the diagnosis and exclude
other causes of renal dysfunction and proteinuria, including numerous
HIV-related glomerular diseases, non-HIV-related renal diseases, and medication
nephrotoxicity. Other glomerular lesions encountered in the HIV-infected
patient include thrombotic microangiopathy, immune complex-mediated glomerular disease (such as membranoproliferative or
membranous glomerulonephritis related to coinfection with hepatitis C or
hepatitis B viruses, acute postinfectious glomerulonephritis, lupus-like
nephritis, and IgA nephropathy). These immune complex forms of
glomerulonephritis are more common in HIV-infected Caucasians than African
Americans. Other renal biopsy findings in the age of antiretroviral therapy
include hypertensive arterio- nephrosclerosis and diabetic nephropathy.
In the acute
phase, untreated HIVAN typically causes a dramatic pattern of collapsing FSGS.
Glomerular capillary lumina are
occluded by an implosive wrinkling and collapse of the glomerular basement
membranes that is more often global than segmental. Tuft collapse is
accompanied by prominent hypertrophy and hyperplasia of the overlying podocytes
(visceral epithelial cells), which have enlarged; open vesicular nuclei with
frequent nucleoli; and occasional mitotic figures. The podocyte cytoplasm is
typically vacuolated, containing intracytoplasmic protein resorption (hyaline)
droplets. Exuberant visceral and parietal epithelial cell proliferation can
form pseudocrescents that obliterate the urinary space. Eventually, the glomerular tuft retracts into a tight,
solidified ball crowned by enlarged, vacuolated visceral epithelial cells.
Tubulo-interstitial
disease is an invariable component of HIVAN and often appears out of proportion
to the degree of glomerular injury. In addition to tubular atrophy,
interstitial fibrosis, edema, and inflammation, there are also widespread tubular
degenerative and regenerative changes, including acute tubular epithelial
injury and hypertrophy with enlarged hyperchromatic nuclei, prominent nucleoli,
mitotic figures, and focal apoptosis. Distended tubules containing loose
protein- aceous casts form tubular microcysts, which may be numerous and
account for the enlarged appearance of the kidneys on radiographic imaging or
gross examination.
By
immunofluorescence, there are no immune complex type deposits. Segmental to
global staining for IgM, C3, and less commonly C1 is frequently observed in the
collapsing segments. These immune reactants are nonspecifically trapped in areas
of sclerosis.
By electron
microscopy, the glomerular capillaries are narrowed by wrinkling and retraction
of the glomerular basement membranes. The overlying podocytes are markedly
hypertrophied with severe foot process effacement, disruption of the actin
cytoskeleton, focal cellular detachment, and intracytoplasmic protein
resorption droplets. No typical immune type of electron dense deposits are
observed. The glomerular endothelial cells contain characteristic
tubulo-reticular inclusions, also known as “interferon footprints.” These 24 nm
structures are located in dilated cisternae of smooth endoplasmic reticulum and
constitute a marker of HIV infection that can be found in endothelial cells and
lymphocytes throughout the body. Importantly, tubulo-reticular inclusions are
not a specific feature of HIVAN and may be found in HIV-infected patients
without nephropathy, as well as in patients with systemic lupus erythematosus,
hepatitis C, or other viral infections. Endothelial tubuloreticular inclusions
have become less common in renal biopsies from patients with HIVAN who are receiving
antiretroviral therapy, consistent with a treatment-induced reduction in viral
burden and associated cytokine dysregulation.
A biopsy
picture of collapsing glomerulopathy is not specific for HIVAN. Differential
diagnosis of the collapsing variant of FSGS includes primary (idiopathic)
FSGS; infections by viruses such as parvovirus B19, SV40 or CMV;
erythrophagocytosis syndrome; interferon therapy; pamidronate toxicity; acute
vasoocclusive injury; and rare familial forms.
TREATMENT
The
introduction of combination antiretroviral therapy in 1996 was followed by a
decline in the incidence of HIVAN and in the number of new cases of ESRD
attributed to HIVAN in the United States. Some case reports demonstrated
histologic improvement of the glomerular collapse and tubular injury on repeat
renal biopsies following antiretroviral therapy, paralleling improvements in
renal function and proteinuria. In addition, antiretroviral therapy has been
found to delay the course of renal failure and prolong renal survival.
Recent
guidelines consider HIVAN an indication for the initiation of antiretroviral
therapy, irrespective of CD4 cell count. Highly active antiretroviral therapy typically includes
combinations of drugs from several classes, including nucleoside and nucleotide reverse transcriptase
inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease
inhibitors. Therapy with ACE inhibitors or angiotensin receptor blockers may be
added to reduce proteinuria and slow disease progression. Corticosteroids have
been used as adjunct therapy in patients with aggressive disease or severe
interstitial inflammation. Patients with HIVAN approaching ESRD can be
maintained on hemodialysis or peritoneal dialysis. Select patients with remote
HIVAN and well-controlled HIV infection are potential candidates for kidney
transplantation.
PROGNOSIS
The natural
history of untreated HIVAN was once rapid progression to ESRD. At present,
however, both proteinuria and renal function can stabilize following
antiretroviral therapy, with relatively slow disease progression. Patients who
develop HIVAN while on anti-retroviral therapy often exhibit a milder form of
FSGS that lacks collapsing
features.