ACUTE INTERSTITIAL
NEPHRITIS
Acute interstitial nephritis (AIN) is a major
cause of intrarenal acute kidney injury (AKI) and features diffuse inflammation
and edema of the tubulointerstitium. It accounts for a small fraction of AKI in
general but is seen in up to 25% of patients with AKI who undergo a renal
biopsy, generally after more common causes (such as prerenal state and acute
tubular necrosis) have been excluded.
PATHOPHYSIOLOGY
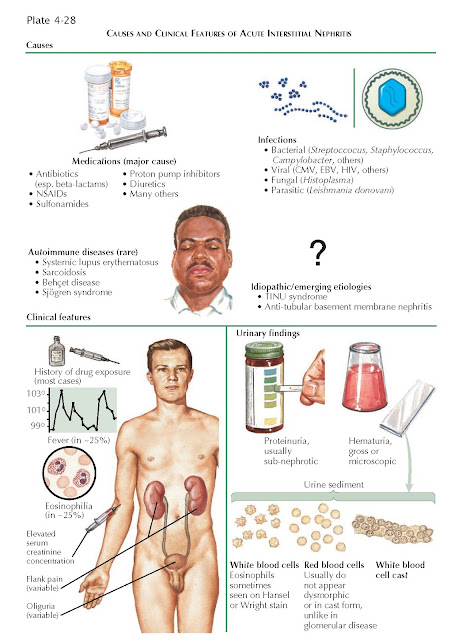
The major known causes of AIN fall
into three broad categories: drugs, infectious diseases, and autoimmune
disorders. Since the implicated drugs and infectious pathogens only cause AIN
in a small fraction of patients, the host’s immune response is likely critical
to the disease pathogenesis.
Drug reactions account for over two
thirds of AIN cases. Although associations with many different drugs have been
reported, the most frequent culprits include β-lactam
antibiotics, rifampin, sulfonamides, diuretics, proton pump inhibitors, and
nonsteroidal anti-inflammatory drugs. In the past, the major cause of
drug-induced AIN was methicillin, which caused disease in up to one in five
patients, but this antibiotic is no longer used in the United States.
Nonetheless, the incidence of drug-induced AIN is rising overall because of
increasing drug use, especially in the older population.
Many drugs cause disease by
inciting a hypersensitivity-type reaction. β-lactams, for example, can serve as haptens by
attaching to proteins on the tubular basement membrane, and forming an antigen
that triggers a T-cell response. NSAIDs, however, appear to trigger disease
through a largely nonallergic mechanism. Although the exact mechanism is
unknown, it has been hypothesized that selective suppression of renal
cyclooxygenase enzymes leads to increased metabolism of arachidonic acids
toward leukotrienes, which trigger an immune response. NSAIDs may also
infrequently induce a hypersensitivity-type response.
Infections account for 15% of AIN
cases, and responsible agents can include bacteria (e.g., Campylobacter,
Salmonella, Streptococcus, Staphylococcus, Escherichia
coli, Brucella), viruses (e.g., cytomegalovirus, Epstein-Barr virus,
HIV, herpes simplex virus), fungi (e.g., Histoplasma), and parasites
(e.g., Leishmania donovani). Such agents can induce inflammation either
through direct invasion of the renal parenchyma or through activation of the
immune system in remote organs with collateral tubulointerstitial involvement.
Infectious agents remain an important cause of AIN in developing nations.
Autoimmune diseases account for 10%
of AIN cases, and responsible diseases include systemic lupus erythematosus,
sarcoidosis, Behçet disease, and Sjögren syndrome.
The remaining AIN cases are
considered idiopathic; however, antitubular basement membrane (TBM) nephritis
and tubulointerstitial nephritis/uveitis (TINU) syndrome are now recognized as
two causes of previously “idiopathic” AIN. Anti-TBM nephritis usually occurs in
early childhood and results from circulating anti-TBM antibodies that target
the proximal tubular basement membrane. TINU syndrome was first described in the
1970s, and a small number of cases has been reported since that time. The
athogenesis is unknown but likely immune-mediated.
PRESENTATION AND DIAGNOSIS
Acute interstitial nephritis
typically manifests as AKI following the recent introduction of a new
medication. Eighty percent of patients develop symptoms within 3 weeks of drug
introduction, although there can be a latent period of several months following
onset of NSAID use. The AKI can manifest either as oliguria or as an
asymptomatic elevation in serum creatinine con-centration noted on routine
serum chemistries. In classic descriptions, the renal injury is
accompanied by the triad of fever, rash, and eosinophilia; however, this
picture emerged when the major pathogenetic agent was methicillin, which often
triggered a hypersensitivity-type reaction. At present, largely because of the
growing incidence of NSAID-related AIN, allergic symptoms are less consistent.
Fever, rash, and eosinophilia are each seen in about 15% to 25% of patients,
and the entire triad is seen in only 10%. In addition to these variable
allergic symptoms, a fraction of patients may experience flank pain,
gross hematuria, or both. Flank pain likely represents distention of the renal
capsule secondary to interstitial edema. Hypertension and gross edema are
uncommon.
Urinalysis often reveals proteinuria,
which is mild on quantitative analysis (i.e., <2
g/day) and reflects impaired tubular reabsorption of filtered proteins.
Nephrotic-range proteinuria may rarely be seen in those cases where NSAID
exposure causes both AIN and minimal change disease (see Plate 4-8).
Microscopic analysis of urine often reveals white blood cells (WBCs), red blood
cells (RBCs), and WBC casts. These findings can facilitate the distinction from
acute tubular necrosis (ATN, see Plate 4-3), which is the most common cause of
intrarenal AKI and typically features a bland sediment or epithelial cell
casts. In addition, the lack of RBC casts or dysmorphic RBCs facilitates the
distinction from acute glomerulonephritis. Finally, the presence of proteinuria
and an active sediment can be used to exclude prerenal state, which may also
occur in the setting of NSAID use secondary to interfe ence with
tubuloglomerular feedback (see Plate 3-18).
Eosinophiluria (defined as
eosinophils >1% of WBCs seen in urine) occurs in some
patients with AIN but can only be detected using special stains, such as Hansel
or Wright stains. Moreover, eosinophiluria may be a non- specific finding because
it can also occur in atheroembolic renal disease, urinary tract infections, and
some glomerulonephritides. Thus its diagnostic utility is unclear.
Renal ultrasound results are often
normal. Diffuse cortical echogenicity secondary to interstitial inflammation has
been described, but no studies have validated the sensitivity or specificity of
this finding. Gallium scan has been proposed as a potentially useful diagnostic
tool. Gallium is a radioactive tracer that colocalizes with WBCs and has
traditionally been used for the detection of abscesses. In acute interstitial
nephritis, there is diffuse, bilateral uptake of gallium, which reflects the
underlying inflammatory process. There are conflicting results, however,
regarding the sensitivity and specificity of this procedure for the diagnosis of
AIN. Thus it is seldom used in clinical practice.
The distinction between AIN or
atheroembolic renal disease can sometimes be challenging because both may cause
AKI with eosinophiluria and mild proteinuria, fevers, arthralgias, and rash.
The rash in AIN, however, is typically maculopapular and erythematous, whereas
atheroembolic disease usually causes livedo reticularis or mottled, violaceous
toes. Atheroembolic disease is also more likely in certain high-risk
populations, such as elderly patients with vascular disease who have recently
undergone an open surgical or percutaneous procedure.
Biopsy is required to confirm the
diagnosis of AIN. It is typically performed in patients with unexplained AKI
and a cellular urine sediment who do not quickly respond to termination of
potentially causative drugs. On light microscopy, AIN features a
lymphocyte-pre-dominant interstitial infiltrate typically accompanied by edema.
The presence of eosinophils suggests a druginduced, allergic cause.
Occasionally, granulomas may also be noted. Tubular injury may occur, with
passage of lymphocytes across the tubular basement membrane (“tubulitis”), but
the glomeruli and blood vessels are typically spared. Inflammation is typically
much more prominent in the renal cortex than in the
medulla.
TREATMENT
Initial treatment for acute
interstitial nephritis includes discontinuation of all potential offending
drugs and eradication of any potential infections. Once an offending drug has
been identified it should never be reintroduced because it will reliably cause
future episodes of interstitial nephritis.
In addition, there is recent
evidence that early steroid administration in drug-induced disease leads to
faster and greater recovery of renal function. Thus, in the absence of any
contraindications, a limited course of corticosteroids may be considered.
PROGNOSIS
Most patients will experience
complete recovery of renal function. A minority will progress to end stage
renal disease and require renal replacement therapy. The duration of renal
failure, rather than the peak serum creatinine concentration, appears to be the
most important indicator of eventual recovery. Some data also suggest patients
of advanced age may have a less favorable prognosis.