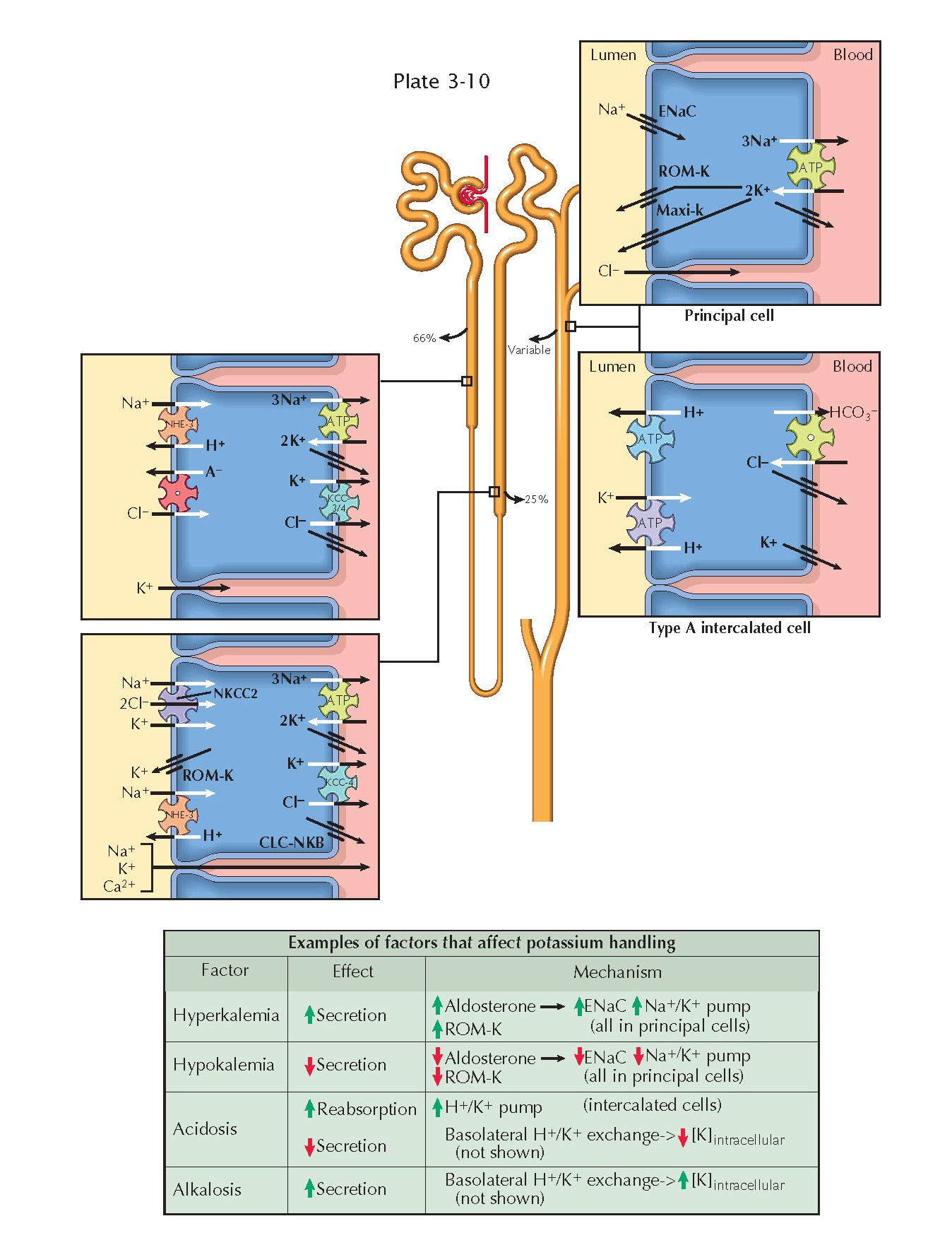
RENAL HANDLING OF POTASSIUM
Potassium is a
primarily intracellular ion, with skeletal muscle
alone containing more than 75% of the body’s total load. Less than 2% of this
load is found in the extracellular fluid. The normal plasma concentration is
between 3.5 and 5.0 mmol/L.
Extracellular potassium is freely filtered at the glomerulus. A large
fraction of the filtered load is consistently reabsorbed along the proximal
tubule (66%) and loop of Henle (25%). In the distal tubule, however, there is a
variable degree of reabsorption or secretion that depends on input from
homeostatic feedback mechanisms. In this manner, the kidneys make a crucial
contribution to plasma potassium concentration.
Transport Mechanisms
Proximal Tubule. In the proximal tubule, potassium is
reabsorbed along a paracellular route. A chemical gradient is established as
the reabsorption of sodium and water concentrates potassium in the tubular
fluid. An electrical gradient is established as chloride is reabsorbed, which
leaves a positive charge in the late part of the proximal tubule. There is some
evidence that potassium also undergoes some transcellular reabsorption in this
segment, but the details and relative importance of this pathway remain
unknown.
Thick Ascending Limb. In the thick ascending limb,
potassium undergoes transcellular reabsorption by crossing the apical membrane
on the Na+/K+/2Cl- cotransporter (NKCC2), then crossing the basolateral
plasma membrane via KCC4 K+/Cl- symporters and potassium channels. A subset
of the potassium that enters the cells, however, is recycled back into the
lumen through ROM-K channels. Such recycling creates a positive charge in the
lumen that drives the paracellular reabsorption of potassium, sodium, and other
cations.
Distal Nephron. Potassium handling is more variable from
the distal convoluted tubule onward, with overall excretion rates depending on
the net balance of secretion and reabsorption.
Secretion occurs primarily in the connecting tubule and cortical
collecting duct. When sodium enters principal cells through apical ENaC
channels, a negative charge is left in the tubular lumen. An electrical
gradient thus established, potassium that has been brought into principal cells
on basolateral Na+/K+ ATPases flows into the lumen through apical
ROM-K and maxi-K (also known as BK) channels. K+ channels are also present in the basolateral compartment, as they
are elsewhere in the nephron, to permit continuous operation of Na+/K+ ATPases.
Reabsorption occurs primarily in the outer medullary collecting duct.
Type A intercalated cells possess apical H+/K+ antiport ATPases, which bring
potassium into cells, and basolateral K+ channels, which allow it to enter the interstitium.
Regulation Of Potassium Excretion
Hyperkalemia promotes the release of aldosterone from the adrenal cortex, which up-regulates
apical ENaC and basolateral Na+/K+
ATPases in principal cells. The resulting
increase in sodium reabsorption enhances the electrical gradient for potassium secretion. Hyperkalemia also causes direct,
aldosterone-independent stimulation of
ENaC and ROM-K channels in principal cells, further
enhancing potassium secretion.
Hypokalemia, in contrast, suppresses aldosterone release and down-regulates apical ROM-K channels in principal cells, thereby reducing
potassium secretion. In addition,
hypokalemia enhances expression of apical H+/K+
ATPases in type A intercalated cells, promoting potassium reabsorption.
Acid-base disturbances also alter potassium secretion or reabsorption, largely because of
basolateral H+/K+ exchange.
In acidosis, protons enter cells to be buffered, and potassium ions exit cells to maintain electroneutrality. The reduction in intracellular potassium
levels decreases the chemical
gradient for secretion into the tubules. In alkalosis,
in contrast, protons exit cells, causing a rise in intracellular potassium levels that promotes secretion.
Finally, volume status has an important relationship with potassium handling. In volume
contraction, AII promotes release of
aldosterone, which enhances potassium secretion.
In volume expansion, increased flow rates
through the nephron stimulate greater potassium secretion through maxi-K channels. Thus potassium secretion is ensured during both volume
expanded and contracted states.