COUNTERCURRENT MULTIPLICATION
The countercurrent multiplier system is a sophisticated apparatus that
evolved in mammals and birds to con-serve water. It forms a longitudinal
concentration gradient in the medullary interstitium that increases in strength
toward the papilla. This gradient is crucial for water reabsorption from the
renal tubules, which is a passive process that depends on osmotic pressure from
the interstitium.
 |
| MODEL OF THE COUNTERCURRENT MULTIPLIER: PART I |
The creation and maintenance of this gradient is best understood by first
considering a simplified model of the loop of Henle. In this model, a tube of fluid is divided by a membrane
in all but its most inferior aspect. The left side represents the entire
descending limb, whereas the right side represents the entire ascending limb.
Fluid enters at the top of the left-sided column, travels beneath the membrane,
and then exits at the top of the right-sided column. The dividing membrane is
impermeable to water but contains active transporters, which pump solute from
the ascending limb to the descending limb. These transporters are powerful
enough to establish a transmembrane gradient of about 200 milliosmoles (mOsm).
In Panel 1, the entire tube is filled with fluid concentrated at 285 mOsm,
which is roughly equal to the osmolality of filtrate as it enters the descending
limb. A transmembrane gradient is established as the transporters pump solute
across the membrane.
In Panel 2, fluid begins to move through the circuit. Thus, at the hairpin
turn, concentrated fluid from the descending limb mixes with less concentrated
fluid from the ascending limb. As a result, a fluid of average concentration is
formed. Because the active transporters can establish a 200 mOsm gradient, the
last part of the descending limb becomes correspondingly more concentrated.
In Panel 3, the flow process continues, and as con-centrated fluid
continues to rise in the ascending limb, reestablishment of the 200 mOsm
transmembrane gradient causes a corresponding rise in the concentration of fluid in the descending
limb. At this stage, solute is still being retained within the system, and thus
the outgoing fluid is less concentrated than the incoming fluid.
In Panel 4, steady state has been reached, meaning that no additional
solute is being added to the system. Thus the incoming and outgoing fluid are
iso-osmotic. The overall effect of this process has been to establish high
longitudinal gradients, whereas the transmembrane gradient is comparatively
small.
In Panel 5, which represents the actual loop of Henle, these same events
occur but with important differences. First, the limbs are separated by an
interstitium, rather than a single membrane. The ascending limb is impermeable
to water but reabsorbs solutes into the interstitium. The descending limb, in
contrast, is permeable to water but not to solutes. As a result, the
concentration of fluid in the descending limb rapidly equilibrates with the
concentration in the interstitium. Another difference is that the fluid leaving
the loop of Henle is hypo-osmotic to the fluid coming in, reflecting the fact
that a small amount of solute is continuously lost from the interstitium,
preventing a steady state from being reached.
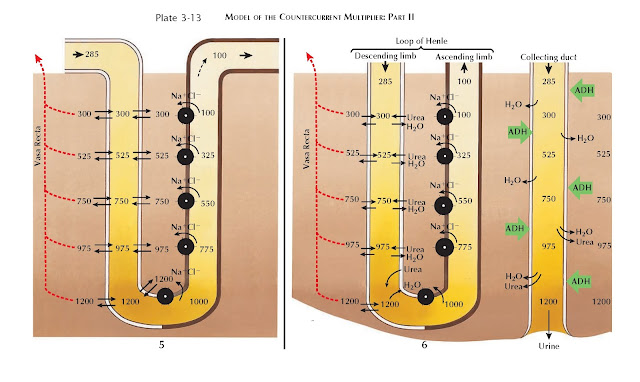 |
| MODEL OF THE COUNTERCURRENT MULTIPLIER: PART II |
In Panel 6, the collecting duct is added to the model and runs parallel
to the loop of Henle. In the presence of ADH (see Plate 3-17), the collecting
duct becomes permeable to water, which is reabsorbed from the col-lecting duct
lumen into the interstitium. This process is entirely passive, depending on the
osmotic pressure of the interstitium. Thus the maximum concentration in the
medullary interstitium determines the maximum concentration of the final urine.
The addition of the collecting duct also illustrates how urea contributes
to formation of the interstitial concentration gradient, especially in the
inner medulla. In the presence of ADH, the inner medullary collecting duct
becomes permeable to urea. As water is reabsorbed from the cortical and outer
medullary collecting ducts, urea becomes highly concentrated within the tubular
fluid. Once the inner medulla is reached, urea flows out of the collecting duct and
accumulates in the interstitium, contributing to the concentration gradient.
Thus, as further described on Plate 3-17, ADH not only promotes water
reabsorption from the collecting duct, but it also activates mechanisms that
strengthen the concentration gradient, thereby ensuring water reabsorption is
maximal.
Once deposited in the interstitium, some urea drifts from the inner
medulla and is secreted back into the proximal tubule and loop of Henle. By
reentering the tubular fluid in this manner, urea is returned to the inner
medullary collecting duct to once again be reabsorbed. This process, known as
urea recycling, tends to minimize urea depletion from the inner medulla.
 |
| MODELS TO DEMONSTRATE PRINCIPLE OF COUNTERCURRENT EXCHANGE SYSTEM OF VASA RECTA IN MINIMIZING DISSIPATION OF MEDULLARY OSMOTIC GRADIENT |
The final elements that need to be added to this model are the capillaries
of the vasa recta, which are permeable to water. If these vessels passed
straight through the interstitium (Panel 7), osmotic pressure would draw out
plasma and dilute the concentration gradient. Instead, the capillaries turn
back upon them-selves (Panel 8), and thus water that effluxes from the descending
capillaries is reabsorbed in the ascending capillaries. This process is known
as countercurrent exchange.
The blood leaving the medulla, however, does not completely reabsorb all
of the effluxed plasma. Thus outgoing blood is slightly hyperosmotic compared
with incoming blood. As a result, the anatomic configuration of the vasa recta
minimizes, but does not completely prevent, solute loss from the medulla. These
losses are also small, because the blood flow to the medulla is very low.
Release of ADH further constricts the vasa recta capillaries, ensuring
maintenance of the high interstitial concentrations required for maximal urine
concentration.




