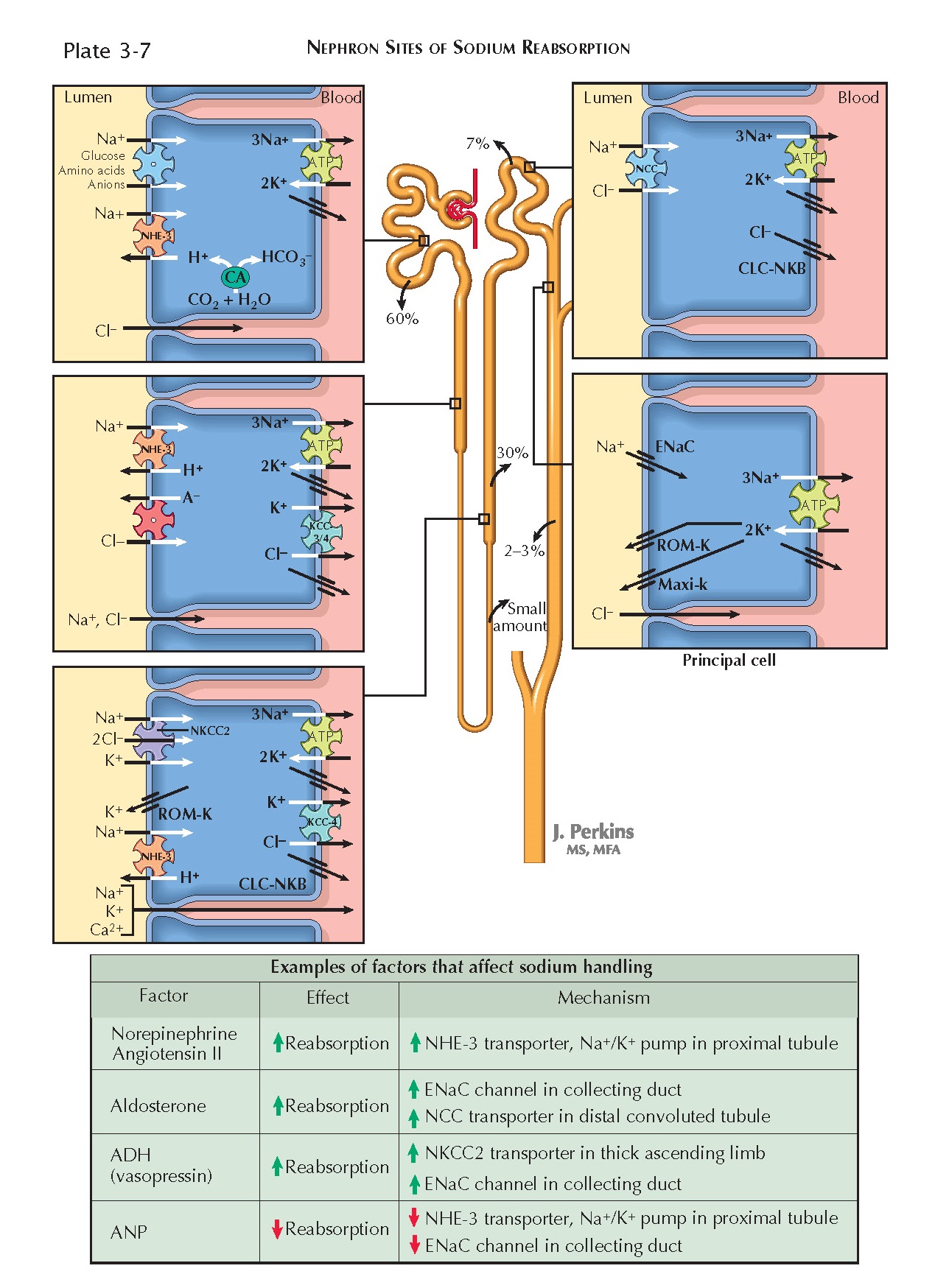
Sodium and chloride are both predominantly extracellular ions. In plasma,
the sodium concentration is maintained between 135 to 145 mmol/L, whereas the
chloride concentration is maintained between 98 to 108 mmol/L.
Both sodium and chloride are freely filtered at the glomerulus and almost
completely (approximately 99%) reabsorbed. 60% of the filtered load is
reabsorbed in the proximal tubule; 30% is reabsorbed in the thick ascending
limb; 7% is reabsorbed in the distal convoluted tubule; and 2% to 3% is
reabsorbed in the connecting tubule and collecting duct.
Mechanisms Of Transport
In all portions of the nephron, basolateral Na+/K+ ATPases pump sodium from the tubular epithelial cells into the
interstitium. As a result, intracellular sodium concentrations remain low,
establishing a gradient for transcellular reabsorption.
Proximal Tubule. Throughout the proximal tubule, sodium
crosses the apical membranes of tubular epithelial cells on Na+/H+ exchangers (NHE-3), causing proton secretion by secondary active
transport. To a lesser extent, sodium crosses apical membranes on symporters
that transport one or more sodium ions in combination with various substances,
including glucose, amino acids, phosphate, lactate, and citrate. The
reabsorption of sodium, irrespective of the mechanism, transiently establishes
an osmotic transepithelial gradient that promotes the passive, isotonic
reabsorption of water (see Plate 3-15).
As sodium and water are reabsorbed, chloride becomes increasingly
concentrated in proximal tubular fluid. In addition, the initial segment of the
proximal tubular lumen has a negative charge. Thus there are chemical and electrical gradients favoring
chloride reabsorption, which occurs
along a paracellular pathway. In later parts of the proximal tubule, the
negative charge in the lumen dissipates, owing to extensive para- cellular
reabsorption of chloride. Instead, there is a positive charge, which creates an
electrical gradient for the paracellular reabsorption of sodium. Despite this
reversal, paracellular chloride reabsorption continues because of the strong
chemical gradient in its favor.
Some chloride also undergoes transcellular reabsorption via apical Cl- anion antiporters, which are coupled with
basolateral Cl- channels and K+/Cl- cotransporters (KCC-3 and -4).
Thin Limb. The descending thin limb is impermeable to
solutes but permits reabsorption of water, as discussed on Plate 3-15. Tubular
fluid thus becomes concentrated in this segment, which establishes a chemical
gradient favoring the reabsorption of some sodium and chloride from the ascending thin limb. Sodium undergoes paracellular reabsorption,
whereas chloride undergoes transcellular reabsorption through apical and
basolateral CLC-NKA channels.
Thick Ascending Limb. In this segment, sodium, chloride,
and potassium undergo transcellular reabsorption together on an apical
cotransporter (NKCC2). Two chloride ions are transported for each sodium and
potassium ion. The basolateral Na+/H+ pumps establish a chemical
gradient for sodium that drives this process. Once in the cell, chloride
crosses the basolateral membrane via channels (CLC-NKB) and K+/Cl- transporters (KCC-4). Potassium, in contrast, is recycled back into
the lumen through apical ROM-K channels. The net result is a positive charge in
the tubular lumen, which promotes the paracellular reabsorption of sodium and other cations.
Although the NHE-3 Na+/H+ exchanger is present in this segment, it
makes only a minor contribution to overall sodium reabsorption and is more
important for bicarbonate reabsorption (see Plate 3-21).
Distal Convoluted Tubule. In this segment, sodium and
chloride undergo transcellular reabsorption together on an apical Na+/Cl- symporter (NCC). The basolateral Na+/K+ pumps establish a chemical gradient for sodium that drives this
process. Once in the cell, chloride crosses the basolateral membrane via the
CLC-NKB channel.
Connecting Tubule and Collecting Duct. In these segments,
sodium undergoes transcellular reabsorption through apical channels (ENaC)
located on principal cells. The reabsorption of sodium generates a negative
charge in the tubular lumen, which creates a gradient for the paracellular
reabsorption of chloride. Although not shown in the illustration, chloride also
undergoes transcellular reabsorption across type B intercalated cells through an apical HCO3-/Cl- exchanger (pendrin) and a
basolateral channel (CLC-NKB).
Regulation Of Sodium Handling
Sodium is the principle osmole of extracellular fluid, and its plasma
concentration is modulated by the systems that control the retention or
excretion of free water. Thus an increase in sodium concentration results in the retention of free water, whereas a
decrease in sodium concentration
results in the excretion of free water. This mechanism is controlled by central
osmoreceptors, which sense increases in osmolality and respond by promoting
feelings of thirst, water-seeking behavior, and the release of antidiuretic
hormone (ADH, or vasopressin). Through water consumption and the actions of
ADH, which promotes water reabsorption from collecting ducts (see Plate 3-17),
free water is added to the extracellular fluid
(ECF) until normal osmolality is
restored. At this point, osmoreceptor activation ceases.
Because of this system, an increase or decrease in total body sodium will
lead, by necessity, to expansion or contraction of the ECF volume. Free water
intake, in contrast, does not affect ECF volume. First, free water distributes
into both the intracellular and extracellular fluids. Second, dilution of the
ECF after fluid intake suppresses ADH release, causing dilute urine to be
produced until normal plasma osmolality is restored. Because total body sodium
is thus the primary determinant of ECF volume, the mechanisms that control ECF
volume directly modulate the rate of sodium excretion
in urine.
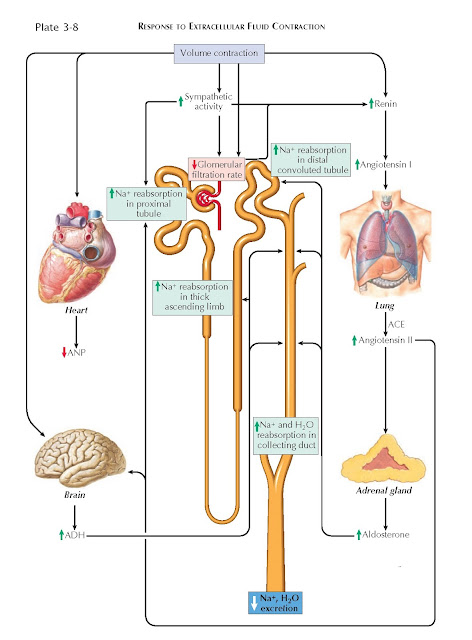
In the setting of ECF depletion, for example, several mechanisms increase
the renal retention of sodium. Activation of baroreceptors in the aortic arch
and carotid bodies, for example, causes an increase in sympathetic tone.
Norepinephrine constricts afferent and efferent arterioles, which reduces the
glomerular filtration rate, and it also stimulates NHE-3 transporters and Na+/K+ ATPases in the proximal tubule, which promotes sodium reabsorption.
Meanwhile, renin release occurs secondary to multiple factors, including
sympathetic input, decreased stretching of afferent arterioles, and decreased
tubular flow rates. Renin catalyzes the synthesis of angiotensin II (AII), which
has many effects that promote sodium retention. First, AII stimulates apical
NHE-3 transporters and basolateral Na+/K+ ATPases in the proximal tubule.
Second, AII promotes the release of aldosterone, which increases sodium
reabsorption from the distal nephron by upregulating ENaC and NCC transporters.
Third, AII promotes the release of ADH, which up-regulates sodium and water
reabsorption from the collecting duct (by up-regulating ENaC and aquaporin channels) and the thick
ascending limb (by upregulating NKCC2
transporters). Finally, AII constricts the efferent arteriole, which lowers
hydrostatic pressure in the peritubular capillaries and, moreover, increases
the filtration fraction, raising osmotic pressure in the peritubular
capillaries. These altered forces both favor reabsorption from the proximal
tubules.
In the setting of ECF overload, these various mechanisms are inactivated,
promoting renal excretion of sodium.
The effect is amplified by the release of atrial natriuretic peptide (ANP), which occurs in response to stretching
of the cardiac atria. ANP dilates the afferent arteriole and constricts the
efferent arteriole, which raises the glomerular filtration rate. In addition, it
blocks sodium reabsorption from the proximal and distal tubules, as well as
water reabsorption from the collecting duct. Finally, it suppresses the release
of renin, aldosterone, and ADH.
 |
| RESPONSE TO EXTRACELLULAR FLUID EXPANSION |