Heart Transplantation: The
Operation
Donor selection
Waiting list mortalit
The appropriateness of using any organ for
transplantation must be balanced against the risk of the recipient dying on the
waiting list if the transplant does not proceed, and in the knowledge that many
other patients who might potentially benefit from transplantation have been
excluded from the list because of donor organ shortage. In the first year on
the waiting list around 60% of patients will receive a heart, while 10–15% will
die waiting.
Suitable hearts
As with all organs, donor sepsis and current
or recent malignancy are contraindications, apart from primary intracranial
malignancy. The heart must be ABO-compatible and lacking HLA anti- gens to
which the recipient has pre-existent antibodies; 30–40% of recipients are
sensitised in this fashion.
Other considerations include the following.
· Donor age: older donors have
an increased burden of coronary artery disease, and donors over 55 years are
seldom used.
· Donor coronary artery disease is associated with early graft failure, unless corrective surgery is
performed to bypass the donor coronary arteries.
· Donor valvular disease or significant left ventricular
hypertrophy. Echocardiography is a
useful way to identify diseased valves (some of which can be repaired) and left
ventricular hypertrophy. A septal thickness of >1.6 cm is a contraindication
to donation.
· Death from carbon monoxide poisoning with carboxyhaemoglobin level above 20%.
· Donor size: aim is to use
hearts from donors with similar weight to recipient; smaller donor hearts and
hearts from females, particularly for recipients with pulmonary hypertension,
tend to fare poorly.
· Left ventricular dysfunction is a common complication of brain stem death, probably related to the
catecholamine storm that occurs. With time and donor fluid management some of
these hearts will recover and be suitable for transplantation; severe dysfunction
(hypokinesia, arrhythmia) is a contraindication.
The donor heart must be macroscopically
examined by the retrieving surgeon. In addition, measurement of cardiac output
and left-side filling pressures, usually with a Swan Ganz catheter is an
essential part of donor assessment.
Transplanting the heart
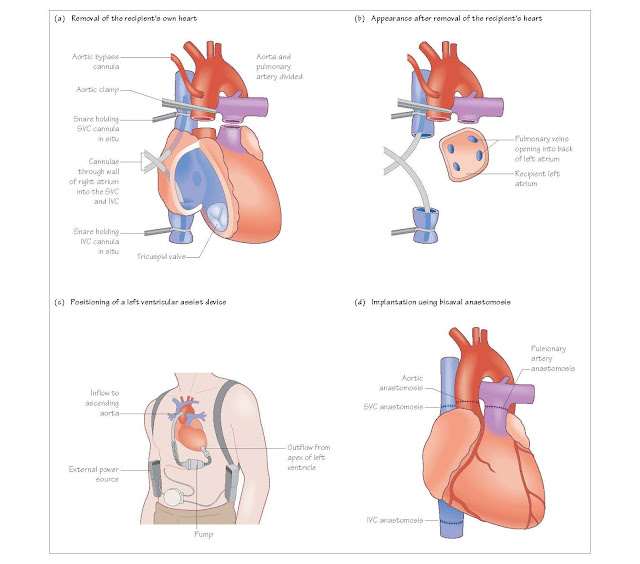
Removing the recipient heart
The heart is approached through a midline
incision dividing the sternum along its length, a median sternotomy. The
recipient is placed on cardiopulmonary bypass so the oxygenation and pump
functions are provided. Cannulae are placed in the ascending aorta and separate
cannulae in the superior and inferior vena cavae.
Blood is then pumped from the vena cavae to
the bypass machine and back into the aortic cannula, and the patient is cooled
to 30°C. When the donor heart is within 20 minutes of the recipient centre the
recipient’s heart is removed. The aorta is cross-clamped and the cavae snared
around the cannulae. The aorta and pulmonary arteries are divided just above
the valves. Both cavae are divided to leave an adequate cuff for sewing to the
donor right atrium. The left atrium is divided along the atrioventricular (AV) groove,
leaving a cuff that contains all the pulmonary veins
Bi-caval implantation technique
The bi-caval implantation technique involves
leaving the donor right atrium intact and instead performing separate
anastomoses with the inferior vena cava (IVC) and superior vena cava (SVC). The
benefits of having a normal size atrium include less atrial dysthyhmia,
pacemaker requirement, tricuspid regurgitation and right ventricular
dysfunction.
Additional considerations
Primary dysfunction of the donor heart is the
cause of most early morbidity and mortality. Ischaemic time is very important.
Mortality increases in a measurable fashion for every hour after the
circulation stops. Good communication is essential between donor and recipient
teams to minimise delays.
Previous cardiac surgery
Patients who have previously undergone a
median sternotomy for cardiac surgery require much longer for cardiectomy, so
it may be necessary to delay the explantation of the donor heart until the
recipient team is ready.
Ventricular assist devices
These are removed at the start of the
recipient procedure, after opening the chest, and may take additional time 2 or
3 hours compared with the 20 minutes it takes to remove a ‘normal’ heart.
Congenital heart disease
The success of neonatal surgery for
congenital heart disease has led to an increasing number of patients coming to
require heart transplantation in later life. Many have unconventional anatomy, clear
delineation of which is essential before surgery. Some procedures, such as in
patients who have had surgery for transposition of the great vessels, may need
cannulation of the femoral vessels instead to facilitate cardiopulmonary
bypass. Additional lengths of aorta and SVC may be needed to cope with
anatomical abnormalities. However, there is no condition, including
dextrocardia, that precludes transplantation.