Endocrine System
Time period: day 24 to birth
Introduction
The glands of the endocrine
system begin to form during the embryonic period and continue to mature during
the foetal period. Functional development can be detected by the presence of
the various hormones in the foetal blood, generally in the second trimester of
pregnancy.
The development of the gonads,
pancreas, kidneys and placenta are covered elsewhere in this book.
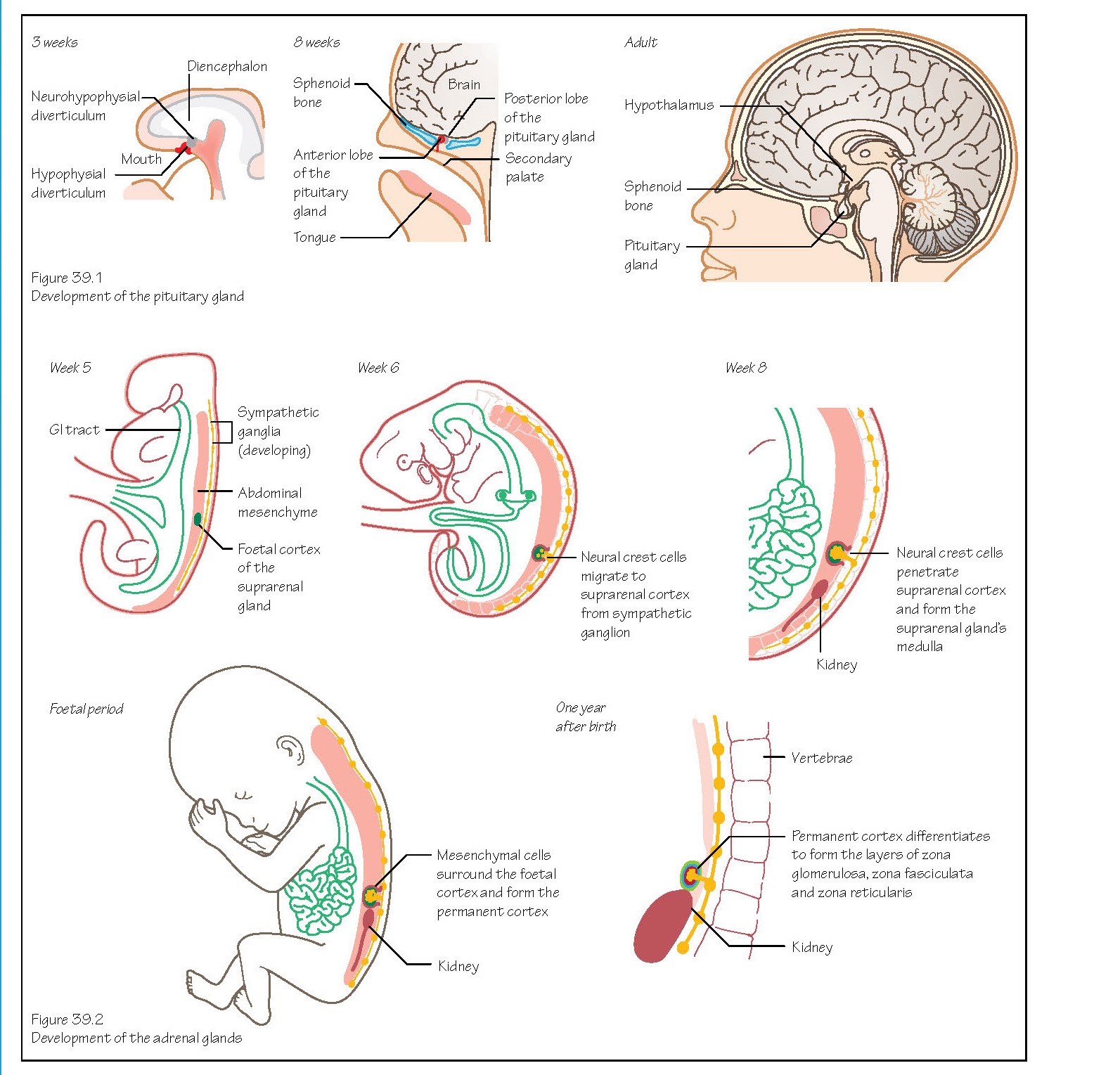
Also known as the hypophysis,
the pituitary gland develops from two sources. An out pocketing of oral
ectoderm appears in week 3 in front of the buccopharyngeal membrane (Figure
39.1). This forms the hypophysial diverticulum (or Rathke’s pouch),
which will become the anterior lobe.
The second source is an extension
of neuroectoderm from the diencephalon, called the neurohypophysial
diverticulum (or infundibulum). The infundibulum grows downwards,
developing into the posterior lobe. These two parts grow towards one another
and by the second month the hypophysial diverticulum is isolated from its
ectodermal origin and lies close to the infundibulum.
Growth hormone secreted by the
pituitary gland can be detected from 10 weeks.
The hypothalamus begins to form
in the walls of the diencephalon (see Chapter 44), with nuclei developing here
that will be involved in endocrine activities and homeostasis.
The pineal body first appears as
a diverticulum in the caudal part of the roof of the diencephalon. It becomes a
solid organ as the cells here proliferate.
The adrenal (or suprarenal)
glands develop from two cell types. The cells of the cortex differentiate
from mesoderm of the posterior abdominal wall near the site of the developing
gonad (Figure 39.2). The adrenaline and noradrenaline secreting cells of the medulla
are derived from migrating neural crest cells that formed a sympathetic
ganglion nearby. These cells become surrounded by the cell mass of the cortex.
The foetal cortex produces a
steroid precursor of oestrogen that is converted to oestrogen by the placenta.
More mesenchymal cells surround the foetal cortex and will become the layers of
the permanent cortex.
The adrenal glands are
exceptionally large in the foetus because of the size of the cortex which
regresses after birth. Substances secreted from the adrenal glands are involved
in the maturation of other systems of the embryo, such as the lungs and
reproductive organs.
This is the first endocrine gland
to develop, beginning at about 24 days between the first and second pharyngeal
pouches from a proliferation of endodermal cells of the gut tube. It begins as
a hollow thickening of the midline where the future tongue will develop. It
eventually becomes solid and then splits into its two lobes.
As the thyroid descends into the
neck it remains connected to the tongue via the thyroglossal duct with
an opening on the tongue called the foramen cecum. The duct degenerates
between weeks 7 and 10 and the thyroid reaches its end location anterior to the
trachea by week 7. If parts of the duct remain the person may also have a pyramidal
lobe. This is quite common and seen in about 50% of the population.
C cells (or parafollicular
cells) are derived from neural crest cells that invade the ultimobranchial
body (a fifth pharyngeal pouch derivative; see Chapter 43).
The inferior parathyroid glands
develop from epithelium (endoderm) of the dorsal wing of the third
pharyngeal pouch. The cells here move with the migration of the thymus
gland into the neck (see Chapter 42). When this connection breaks down they
become located on the dorsal surface of the thyroid gland.
Endoderm cells of the dorsal wing
of the fourth pharyngeal arch begin to collect and differentiate to form
the superior parathyroid glands (initially the superior parathyroid glands are
inferior to the inferior parathyroid glands). These cells are associated with
the developing thyroid gland and migrate with it, but for a shorter distance
than the cells of the inferior parathyroid glands (see Chapter 43). They also
rest on the dorsal surface of the thyroid, but generally more medially and
posteriorly.
Clinical relevance
Pituitary gland
Congenital hypopituitarism is
a decrease in the amount of one or more of the hormones secreted by the
pituitary gland. Symptoms are wide ranging, depending upon which hormones are
affected. The cause is often hypoplasia of the gland or complications with
delivery. Treatment is commonly oral or injection replacement of the
insufficient hormones.
Adrenal glands
Congenital adrenal hyperplasia is an autosomal recessive disease causing excessive production of
steroids, with 95% of patients deficient in the enzyme 21‐hydroxylase (required
in the production of adrenal secretions). There are degrees of severity and
this can cause ambiguous genitalia and infertility. Various treatment options
are available and can include glucocorticoids, sex hormone replacement and
genital reconstructive surgery.
Thyroid gland
Congenital hypothyroidism is
a deficiency in thyroid hormone production. Symptoms include excessive sleeping
and poor feeding. Newborn infants are screened for this and if this deficiency
is found treatment is a daily thyroxine tablet.
Ectopic thyroid tissue left
behind during migration is relatively common but asymptomatic. Parts of the
thyroglossal duct may persist and form a midline, moveable cyst in a child.
Parathyroid glands
Hypoparathyroidism is an
absence of parathyroid hormone. Symptoms are wide ranging but often not
diagnosed until 2 years of age. They include seizures and poor growth.
Treatment includes vitamin D and calcium supplements.
Ectopic parathyroid tissue left
behind during migration is relatively common but asymptomatic more common for the inferior parathyroid glands.