The Structure and Function of The Immunoglobulin Classes
The
immunoglobulin classes (Table 3.1) fulfill different roles in immune defense
and this can be correlated with differences in their structures as organized
around the four‐chain Ig domain arrangement (Figure 3.12). IgG is
monomeric and the major antibody in serum and nonmucosal tissues, where it
inactivates pathogens directly and through interaction with effector triggering
molecules, such as complement and Fc receptors. IgM is
pentameric, is found in serum, and is highly efficient at complement triggering.
A monomeric form of IgM with a membrane‐tethering sequence is the major
antibody receptor used by B‐lymphocytes to recognize antigen (see Figure 2.11).
IgM differs from IgG in having an extra pair of constant domains instead of the
hinge region. IgA exists in three soluble forms. Monomeric and
small amounts of dimeric IgA (formed from two monomers linked by an extra
polypeptide called J chain) are found in the serum where they can help link
pathogens to effector cells via Fc receptors specific for IgA. Secretory IgA is
formed of dimeric IgA and an extra protein known as secretory component (SC)
and is crucial in protecting the mucosal surfaces of the body against attack by
microorganisms. IgA exists as two subclasses in humans. IgA2 has a much shorter
hinge than IgA1 and is more resistant to attack by bacterially secreted
proteases. IgE is a monomeric antibody typically found at very
low concentrations in serum. In fact, most IgE is probably bound to IgE Fc
receptors on mast cells. Antigen binding to IgE crosslinks IgE Fc receptors and
triggers an acute inflammatory reaction that can assist in immune defense. This
can also lead to unwanted allergic symptoms for certain antigens (allergens).
IgE, like IgM, has an extra pair of constant domains instead of the hinge
region. Finally, IgD is an antibody primarily found on the
surface of B‐cells as an antigen receptor together with IgM, where it likely
serves in the control of lymphocyte activation and suppression. There is also
some evidence that free IgD may help protect against microbes in the human
upper respiratory tract. IgD is monomeric and has a long hinge region.
The
structures of the Fc regions of human IgA1 and IgE have been determined and are
compared with IgG1 in Figure 3.13. In all three cases, the penultimate domains
are unpaired and have carbohydrate chains interposed between them.
 |
Figure 3.12 Schematic
structures of the antibody classes. The two heavy chains are shown in dark and
pale blue (two colors to highlight chain pairing; the chains are identical) and
the light chains in gray. The N‐linked carbohydrate chains (branched
structures) are shown in blue and O‐linked carbohydrates (linear
structures) in green. The heavy chain domains are designated according to the
class of the heavy chain (e.g., Cγ2 for the CH2 domain of IgG, etc.). For IgG,
IgA, and IgD, the Fc is connected to the Fab arms via a hinge region; for IgM
and IgE an extra pair of domains replaces the hinge. IgA, IgM, and IgD have
tailpieces at the C‐termini of the heavy chains. IgA occurs in monomer and dimer
forms. IgM occurs as a pentamer. (a) IgG1. The other human IgG subclasses (and
IgGs of most other species) have this same basic structure but differ
particularly in the nature and length of the hinge. (b) IgA1. The structure
resembles IgG1 but with a relatively long hinge bearing O‐linked sugar
chains. The Fc also shows some differences from IgG1 (see Figure 3.13). In
IgA2, the hinge is very short and, in the predominant allotype, the light
chains are disulfide linked not to the heavy chain but to one another. (c) IgM
monomeric unit. This representation relies greatly on comparison of the amino
acid sequences of μ and γ heavy chains. (d) IgE. The molecule is similar to the
monomeric unit of IgM. (e) IgD. The hinge can be divided into a region rich in
charge (possibly helical) and one rich in O‐linked sugars. The structure
of the hinge may be much less extended in solution than represented
schematically here. It is, however, very sensitive to proteolytic attack so
that serum IgD is unstable. Mouse IgD has a structure very different to that of
human IgD, in contrast to the general similarity in structures for human and
mouse Igs. (f) Secretory IgA (see also Figure 3.19). (g) Pentameric IgM. The
molecule is represented as a planar star shape. One monomer unit is shown
shaded as in (c). A minority of IgM units can also form a hexamer. For clarity
the carbohydrate structures have been omitted in (f) and (g). The Fab arms can
likely rotate out of the plane about their two‐fold axis (see also Figure
3.14).
 |
Figure 3.13 The
structures of the Fc regions of human IgG1, IgE, and IgA1. The structures shown
were determined by crystallographic analysis of Fcs in complex with Fc
receptors. One heavy chain is shown in red, the other in yellow and the
N‐linked carbohydrate chains that are interposed between the penultimate
domains are shown in blue. For IgE, the Fc structure is shown for the C ε4 –C ε3
domain fragment for comparison;. a structure is now available including the C ε2
domains. For IgA1, the N‐linked sugars are attached at a position quite
distinct from that for IgG1 and IgE. Also the tips of the Cα2 domain are joined
by a disulfide bridge. (Source: Woof J.M. and Burton D.R. (2004) Nature
Reviews Immunology 4, 89–99. Reproduced with permission of Nature
Publishing Group.)
Antibodies and complement
The
clustering together of IgG molecules, typically on the surface of a pathogen
such as a bacterium, leads to the binding of the complement C1 molecule via the
hexavalent C1q subcomponent (see Figure 2.2). This triggers the classical
pathway of complement and a number of processes that can lead to pathogen
elimination. Recently, it has been proposed that the most favorable clustered
arrangement of antibodies on an antigen surface for complement triggering may
also be hexameric, thereby matching the symmetry of C1q. The subclasses of IgG
trigger with different efficiencies. IgG1 and IgG3 trigger best; IgG2 is only
triggered by antigens at high density (e.g., carbohydrate antigens on a
bacterium); and IgG4 does not trigger.
IgM triggers
by a different mechanism. It is already “clustered” (pentameric) but occurs in
an inactive form. Binding to multivalent antigen appears to alter the
conformation of the IgM molecule to expose binding sites that allow C1q to bind
and the classical pathway of complement to be triggered. Electron microscopy
studies suggest the conformational change is a “star” to “staple” transition,
in which the Fab arms move out of the plane of the Fc regions (Figure 3.14).
IgM antibodies tend to be of low affinity as measured in a univalent
interaction (e.g., binding of IgM to a soluble monomeric molecule or binding of
an isolated Fab from an IgM to an antigen). However, their functional affinity
(avidity) can be enhanced by multivalent antibody antigen interaction and it is
precisely under such circumstances that they are most effective at activating
complement.
 |
Figure 3.14 Structural
changes in IgM associated with complement activation. (a) The “star”
conformation. Electron micrograph of an uncomplexed IgM protein shows a “star‐
shaped” conformation (see Figure 3.12 g). (b) The “staple” conformation.
Electron micrograph of a specific sheep IgM bound to a Salmonella paratyphi flagellum
as antigen suggests that the five F(ab′)2 units and Cμ2 domains have been
dislocated relative to the plane of the Fcs to produce a “staple” or
“crab‐like” conformation. Complement C1 is activated on binding to
antigen‐complexed IgM (staple), but interacts only very weakly, yielding no
significant activation, with free IgM (star), implying that the dislocation
process plays an important role in complement activation. It is suggested that
movement of the Fabs exposes a C1q‐binding site on the Cμ3 domains of IgM. This
is supported by observations that an Fc5 molecule, obtained by papain digestion
of IgM, can activate complement directly in the absence of antigen. Electron
micrographs are negatively stained preparations of magnification × 2 × 106,
i.e., 1 mm represents 0.5 nm. (Source: Dr. A. Feinstein and Dr. E.A. Munn.
Reproduced with permission.)
Antibodies and human leukocyte Fc
receptors
Specific
human Fc receptors have been described for IgG, IgA, and IgE (Table 3.2). The
receptors differ in their specificities for antibody classes and subclasses,
their affinities for different association states of antibodies (monomer versus
associated antigen‐complexed antibody), their distributions on different
leukocyte cell types, and their cellular signaling mechanisms. Most of the
leukocyte Fc receptors are structurally related, having evolved as members of
the Ig gene superfamily. Each comprises a unique ligand‐binding chain (α
chain), which is often complexed via its transmembrane region with a dimer of the
common FcRγ chain. The latter plays a key role in the signaling functions
of many of the receptors. FcRγ chains carry immunoreceptor
tyrosine‐based activation motifs (ITAMs) in their cytoplasmic regions, critical
for initiation of activatory signals. Some receptor α chains carry their own
ITAMs in their cytoplasmic regions, whereas others bear the immunoreceptor
tyrosine‐based inhibitory motifs (ITIMs).
For IgG,
three different classes of human leukocyte FcγRs have been characterized, most
with several variant forms. In addition, the neonatal Fc receptor FcRn also
binds IgG and will be dealt with later. Fc γRI (CD64) is
characterized by its high affinity for monomeric IgG. It is also unusual in
that it has three extracellular Ig‐like domains in its ligand‐binding chain,
while all other Fc receptors have two. FcγRI is constitutively expressed on
monocytes, macrophages and dendritic cells, and is induced on neutrophils and
eosinophils following their activation by IFNγ and G‐CSF (granulocyte
colonystimulating factor). Conversely, FcγRI can be downregulated in response
to IL‐4 and IL‐13. Structurally, it consists of an IgG‐binding α chain and a γ
chain homodimer containing ITAMs. It binds monomeric IgG avidly to the surface
of the cell, thus sensitizing it for subsequent encounter with antigen. Its
main roles are probably in facilitating phagocytosis, in antigen presentation,
and in mediating extracellular killing of target cells coated with IgG
antibody, a process referred to as antibody‐dependent cellular cytotoxicity
(ADCC).
Fc γRII (CD32) binds very weakly
to monomeric IgG but with considerably enhanced affinity to associated IgG, as
in immune complexes or on an antibody‐coated target cell. Therefore, cells
bearing FcγRII are able to bind antibodycoated targets in the presence of high
serum concentrations of monomeric IgG. Unlike the single isoform of FcγRI,
there are multiple expressed isoforms of FcγRII that collectively are present
on the surface of most types of leukocyte (Table 3.2). The binding of IgG complexes
to FcγRII triggers phagocytic cells and may provoke thrombosis through their
reaction with platelets. FcγRIIa are activating receptors expressed on
phagocytes that mediate phagocytosis and ADCC. In contrast, FcγRIIb are
inhibitory receptors that have cytoplasmic domains containing ITIMs and their
occupation leads to downregulation of
cellular responsiveness.
FcγRIIb occurs as
two isoforms generated by
alternative splicing. FcγRIIb1 present on B‐cells cross‐links B‐cell receptors
(BCR) and transmits an inhibitory signal to inactivate the B‐cell with a
negative‐feedback effect on antibody production. FcγRIIb2 is expressed on
phagocytes, where it efficiently mediates endocytosis, leading to antigen
presentation.
Fc γRIII (CD16) also binds rather
poorly to monomer IgG but has low to medium affinity for aggregated IgG. The
two FcγRIII genes encode the isoforms FcγRIIIa and FcγRIIIb that have a medium
and low affinity for IgG, respectively. FcγRIIIa is found on most types of
leukocyte, whereas FcγRIIIb is restricted mainly to neutrophils and is unique
among the Fc receptors in being attached to the cell membrane by a
glycosylphosphatidylinositol (GPI) anchor rather than a transmembrane segment.
FcγRIIIa is known to be associated with the γ chain signaling dimer on
monocytes and macrophages, and with either ζ and/or γ chain signaling molecules
in NK cells, and its expression is upregulated by transforming growth factor β
(TGFβ) and downregulated by IL‐4. With respect to their functions, FcγRIIIa is
largely responsible for mediating ADCC by NK cells and the clearance of immune
complexes from the circulation by macrophages. For example, the clearance of
IgG‐ coated erythrocytes from the blood of chimpanzees was essentially
inhibited by the monovalent Fab fragment of a monoclonal anti‐FcγRIII. FcγRIIIb
cross‐linking stimulates the production of superoxide by neutrophils.
For IgE, two
different FcγRs have been described. The binding of IgE to its receptor Fc
εRI is characterized by the remarkably high affinity of the
interaction, reflecting a very slow dissociation rate (the half‐life of the
complex is ∼ 20 hours). Fc εRI is a
complex comprising a ligand‐binding α chain struc turally related to those of
FcγR, a β chain, and the FcRγ chain dimer. Contact with antigen leads to
degranulation of the mast cells with release of preformed vasoactive amines and
cytokines, and the synthesis of a variety of inflammatory mediators derived
from arachidonic acid (see Figure 1.14). This process is responsible for the
symptoms of hay fever and of extrinsic asthma when patients with atopic allergy
come into contact with the allergen (e.g., grass pollen). The main
physiological role of IgE would appear to be protection of anatomical sites
susceptible to trauma and pathogen entry by local recruitment of plasma factors
and effector cells through the triggering of an acute inflammatory
reaction. Infectious agents penetrating the IgA defenses would combine
with specific IgE on the mast cell surface and trigger the release of
vasoactive agents and factors chemotactic for polymorphs, so leading to an
influx of plasma IgG, complement, neutrophils, and eosinophils. In such a
context, the ability of eosinophils to damage IgG‐coated helminths and the
generous IgE response to such parasites would constitute an effective defense.
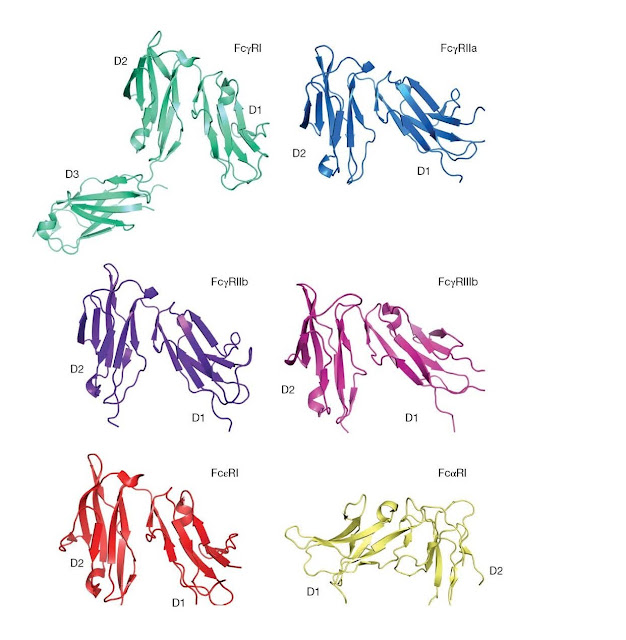 |
Figure 3.15 Structures
of human leukocyte Fc receptors. In each case, a similar view of the receptor
is shown. D1, membrane distal; D2, membrane‐proximal domain, except for FcγRI
for which D3 is the proximal domain and FcαRI for which D2 is membrane
proximal. For the FcγRs and Fc εRI, the Fc‐binding site is present at the “top”
of the D2 domain, whereas for FcαRI the Fc‐interaction site is present at the
top of the D1 domain. (Source: Jenny Woof and Christina Corbaci. Reproduced
with permission.)
The
low‐affinity IgE receptor FcεRII (CD23) is a C‐type (calcium‐dependent)
lectin. It is present on many different types of hematopoietic cells (Table
3.2). Its primary function appears to be in the regulation of IgE synthesis by
B‐cells, with a stimulatory role at low concentrations of IgE and an inhibitory
role at high concentrations. It can also facilitate phagocytosis of
IgE‐opsonized antigens.
For IgA, FcαRI
(CD89), is the most well‐characterized Fc receptor. Its ligand‐binding
α chain is structurally related to those of the FcγRs and FcεRI but represents a
more distantly related member of the family. In fact, it shares closer homology
with members of a family including NK cell immunoglobulin‐ like receptors
(KIRs), leukocyte Ig‐like receptors (LIR/LILR/ILTs) and the platelet‐specific
collagen receptor (GPVI). FcαRI is present on monocytes, macrophages,
neutrophils, eosinophils, and Kupffer cells. The cross‐linking of FcαRI by
antigen can activate endocytosis, phagocytosis, inflammatory mediator release,
and ADCC. Expression of FcαRI on monocytes is strongly upregulated by bacterial
polysaccharide.
Crystal
structures are available for FcγRIa, FcγRIIa, FcγRIIb, FcγRIIIb, FcεRI, and
FcαRI (Figure 3.15). In most cases, the structures represent the two Ig‐like
extracellular domains of the receptor α chain, termed D1 (N‐terminal, membrane
distal) and D2 (C‐terminal, membrane proximal). No structure is yet available
for the cytoplasmic port f any receptor. The equivalent extracellular regions
of I,
FcγRIIa/b,
FcγRIII, and Fc εRI are seen to share the same overall structure. Despite the
basic sequence similarity between FcαRI and these receptors, the IgA receptor
turns out to have a strikingly different structure. Although the two individual
domains of the FcαRI extracellular portion fold up in a similar manner to those
of the other receptors, the arrangement of the domains relative to each other
is very different. The domains are rotated through ∼ 180° from the positions adopted in the other Fc
receptors, essentially inverting the D1–D2 orientations. The Fc εRII receptor
also has a different structure altogether: its lectin‐like head domain binds
between the C ε2 and C ε3 domains of IgE Fc.
Crystallographic
studies of antibody–Fc receptor complexes have revealed how antibodies interact
with leukocyte Fc recep tors (Figure 3.16). For the IgG–FcγRIII interaction,
the D2 membrane‐proximal domain of FcγRIII interacts with the top of the CH2
domains and the bottom of the hinge. This requires the antibody to adopt a
“dislocated” conformation in which the
Fab arms are
rotated out of the plane of the Fc. One consequence of this mode of
interaction, recognized many years ago, is that it promotes close approach of
the target cell membrane (upwards on the page) to the effector cell membrane.
This may favor effector cell activity against the target cell. Given the
similarities between FcγRI, FcγRII, and FcγRIII, it is likely that all three
FcRs share a common mode of binding to IgG. Indeed, this mode of binding seems
also to be shared by IgE binding to the Fc εRI receptor, although the Cε2–C ε3
domain linker region replaces the hinge contribution to receptor binding. By
contrast, IgA binds to the FcαRI receptor at a site between Cα2 and Cα3
domains. This mode of binding permits an IgA:FcR stoichiometry of 2 : 1,
whereas the stoichiometry for IgG and IgE in these complexes is 1 : 1. The
significance of these differences in the modes of binding is not understood at
this time.
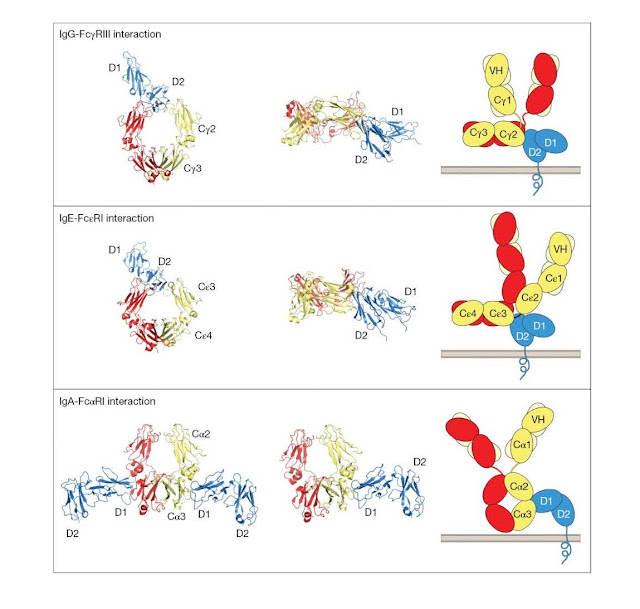 |
Figure 3.16 Structures
of antibody–leukocyte Fc receptor interactions. The left‐hand side and middle
columns show views of the crystal structures of the complexes of the FcRs with
their respective Fc ligands. The extracellular domains of the receptors are
shown in blue; one heavy chain of each Fc region is shown in red and the other
in dark yellow. In the left‐hand column, each Fc region is viewed face on. The
similarity between the IgG–FcγRIII and IgE–Fc εRI interactions is striking,
whereas the IgA–FcαRI interaction is quite different in terms of the sites
involved and the stoichiometry. The middle column shows a view where the D2
domains of each of the receptors are positioned so that their C‐termini face
downwards. Here the Fc regions of IgG and IgE are seen in a horizontal position
from the side. For the IgA interaction only one receptor molecule is shown. The
right‐hand column shows a schematic representation of the receptors and their
intact ligands from the same viewpoint as the images in the middle column.
Light chains are shown in pale yellow. The necessity for dislocation of IgG and
IgE to allow positioning of the Fab tips away from the receptor‐bearing cell
surface is apparent. (Source: Jenny Woof and Christina Corbaci. Reproduced with
permission.)
 |
Figure 3.17 Function
of the neonatal receptor for IgG (FcRn). (a) The FcRn receptor is present on
the syncytiotrophoblast of the placenta where it fulfills the important task of
transferring maternal IgG to the fetal circulation. This will provide
protection prior to the generation of immunocompetence in the fetus.
Furthermore, it is self‐evident that any infectious agent that might reach the
fetus in utero will have had to have passed through the mother first,
and the fetus will rely upon the mother’s immune system to have produced IgG
with appropriate binding specificities. This maternal IgG also provides
protection for the neonate, because it takes some weeks following birth before
the transferred IgG is eventually all catabolized.
Antibodies and the neonatal Fc
receptor
An important
Fc receptor for IgG is the neonatal receptor, FcRn. This receptor mediates transport
of IgG from mother to child across the placenta (Figure 3.17a). Such
antibody, surviving for some time in the blood of the newborn child, is
believed to be important in directly protecting the child from pathogens.
Furthermore, the presence of maternal antibody has been proposed to help the
development of cellular immunity in the young child by attenuating pathogen
challenge rather than stopping it completely. FcRn may also be important in
transporting maternal IgG from mother’s milk across the intestinal cells of the
young infant to the blood. Equally, FcRn is crucial in maintaining the
long half‐life of IgG in serum in adults and children. The receptor
binds IgG in acidic vesicles (pH < 6.5), protecting the molecule from
degradation, and then releasing the IgG at the higher pH of 7.4 in blood
(Figure 3.17b). This constant recycling of IgG and prevention of degradation in
endosomes increases the half‐life of IgG relative to other antibody isotypes.
FcRn has a number of other important functions, including facilitating antigen
presentation for antigens derived from the gut, transport of IgG into a number
of secretions, and regulation of serum albumin persistence.
Structural
studies have revealed the molecular basis for FcRn activity. FcRn is unlike
leukocyte Fc receptors and instead has structural similarity to MHC class I
molecules. It is a hetero dimer composed of a β2‐microglobulin chain
noncovalently attached to a membrane‐bound chain that includes three
extracellular domains. One of these domains, including a carbohydrate chain,
together with β2‐microglobulin interacts with a site between the CH2
and CH3 domains of Fc (Figure 3.18). The interaction includes three
salt bridges made to histidine (His) residues on IgG that are positively
charged at pH < 6.5. At higher pH, the His residues lose their positive
charges, the FcRn–IgG interaction is weakened and IgG dissociates.
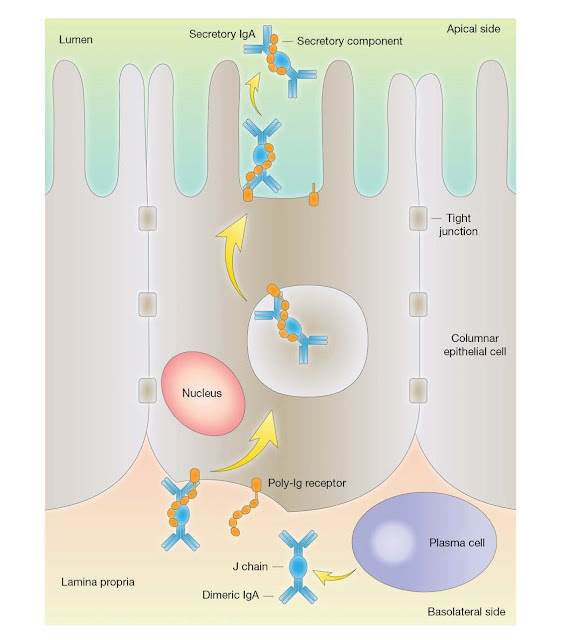
Secretory IgA
IgA appears
selectively in the seromucous secretions, such as saliva, tears, nasal fluids, sweat,
colostrum, milk, and secretions of the lung, genitourinary, and
gastrointestinal tracts, where it defends the exposed external surfaces of the
body against attack by microorganisms. This is an important function as
approximately 40 mg of secretory IgA/kg body weight is transported daily
through the human intestinal crypt epithelium to the mucosal surface as
compared with a total daily production of IgG of 30 mg/kg.
The IgA is
synthesized locally by plasma cells and dimerized intracellularly together with
a cysteinerich polypeptide called J chain, of molecular weight 15 000. Dimeric
IgA binds strongly to a receptor for polymeric Ig (poly‐Ig receptor (pIgR),
which also binds polymeric IgM) present in the membrane of mucosal epithelial
cells. The complex is then actively endocytosed, transported across the
cytoplasm, and secreted into the external body fluids after cleavage of the
pIgR peptide chain. The fragment of the receptor remaining bound to the IgA is
termed secretory component and the whole molecule, secretory IgA (Figure
3.19).
Isotypes, allotypes, and idiotypes:
antibody variants
The
variability of antibodies is often conveniently divided into three types:
isotypes, allotypes, and idiotypes. Isotypes are variants present
in all healthy members of a species: immunoglobulin classes and subclasses are
examples of isotypic variation involving the constant region of the heavy
chain. Allotypes are variants that are inherited as alternatives
(alleles) and therefore not all healthy members of a species inherit a
particular allotype. They occur mostly as variants of heavy chain constant
region genes, in humans in all four IgG subclasses, IgA2, and IgM. The nomenclature
of human immunoglobulin allotypes is based on the isotype on which it is found
(e.g., G1m defines allotypes on an IgG1 heavy chain, Km defines allotypes on k
light chains) followed by an accepted World Health Organization (WHO) numbering
system.
The variable
region of an antibody can act as an antigen, and the unique determinants of
this region that distinguish it from most other antibodies of that species are
termed its idiotypic determinants. The idiotype of an antibody,
therefore, consists of a set of idiotypic determinants that individually are
called idiotopes. Polyclonal anti‐idiotypic antibodies generally recognize a
set of idiotopes, whereas a monoclonal anti‐idiotype recognizes a single
idiotope. Idiotypes are usually specific to an individual antibody clone
(private idiotypes) but are sometimes shared between different antibody clones
(public, recurrent, or cross‐reacting idiotypes). An anti‐idiotype may react
with determinants distant from the antigen‐binding site, it may fit the binding
site and express the image of the antigen, or it may react with determinants
close to the binding site and interfere with antigen binding. Sequencing of an
anti‐idiotypic antibody generated against an antibody specific for the
polypeptide GAT antigen in mice revealed a CDR3 with an amino acid sequence
identical to that of the antigen epitope (i.e., the anti‐idiotype contains a
true image of the antigen) but this is probably the exception rather than the
rule.