Energy Homoeostasis Summary
Clinical background
In recent years, adipose tissue has
become recognized as a highly metabolically active organ. In 1994, the hormone
leptin was identified, a peptide almost exclusively secreted by adipose cells
and with receptors both in the hypothalamus and peripheral tissues. Leptin has
a number of actions both in relation to signalling satiety and altering energy
metabolism. The identification of a rare family with leptin deficiency, extreme
obesity and insulin resistance was followed by treatment of two children with
recombinant leptin and successful loss of weight. However, in the majority of
non-leptin-deficient obese individuals, circulating leptin levels are high and
correlated with body fat mass, suggesting that leptin resistance may play a
role in human obesity. Further work is needed to establish the exact role of
this hormone in energy homeostasis.
Endocrine hormones and energy
metabolism
The neuroendocrine system plays a
critical role in energy metabolism and homeostasis and is implicated in the
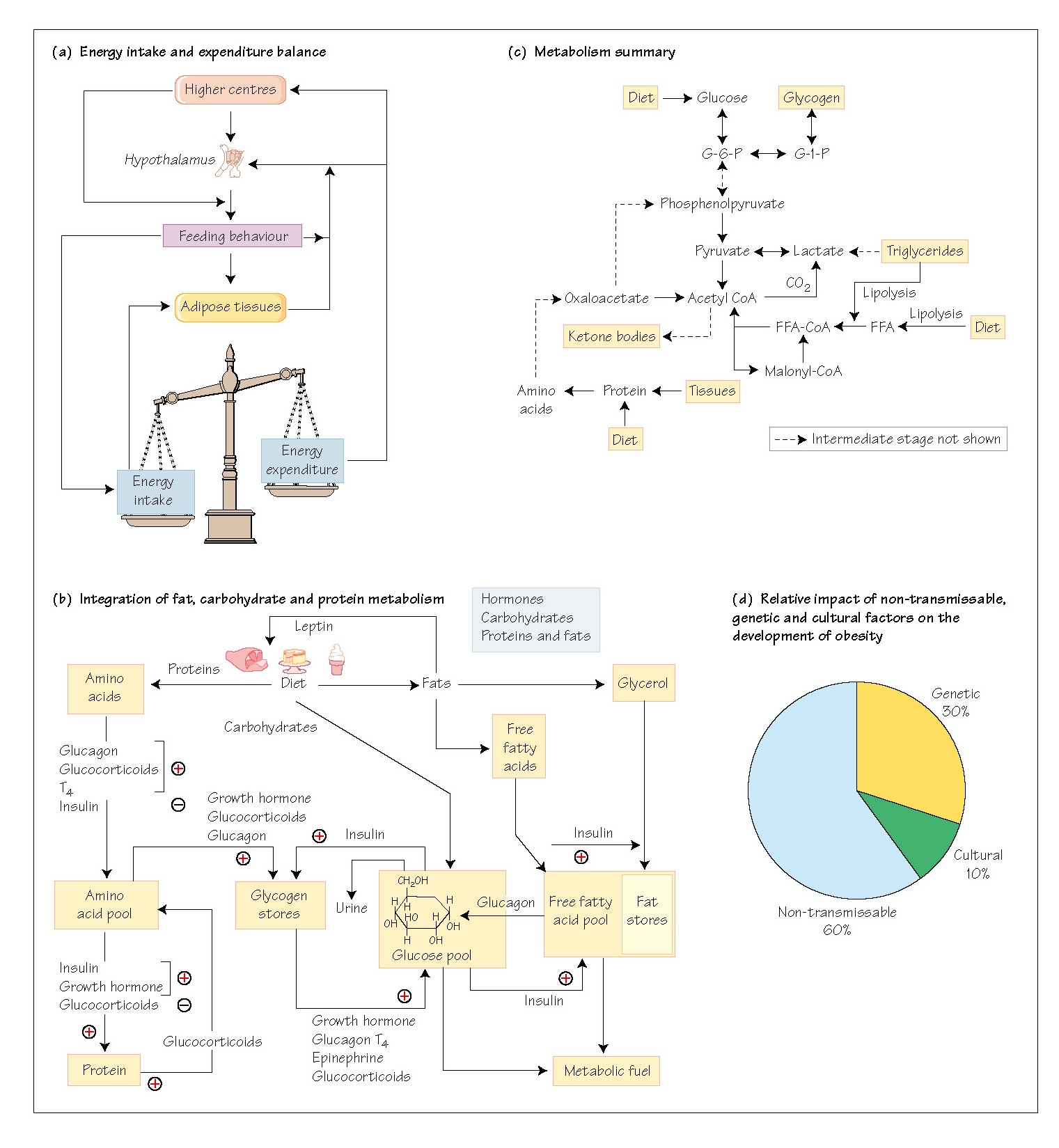
control of feeding behaviour. Energy metabolism centres on the maintenance of
an adequate supply of glucose for metabolism and on the balance between energy
storage and utilization (Fig. 44a). The rapid spread of obesity, with attendant
diabetes and heart disease, in western affluent societies has promoted research
that has identified previously unknown endocrine hormones that regulate, and
indeed dictate, feeding behaviour in other species (see below and Chapter 45).
Energy stores
Fats are the main energy stores in the
body. Fats provide the most efficient means of storing energy in terms of kJ/g,
and the body can store seemingly unlimited amounts of fat, a fact evident from
the phenomenon of extreme obesity. Carbohydrate constitutes <1% of energy
stores, and tissues such as the brain are absolutely dependent on a constant
supply of glucose, which must be supplied in the diet or by gluconeogenesis.
Proteins contain about 20% of the body’s energy stores, but since proteins have
a structural and functional role, their integrity is defended, except in
fasting, and these stores are therefore not readily available.
Circulating glucose can be considered
as a glucose pool (Fig. 44b), which is in a dynamic state of equilibrium,
balancing the inflow and outflow of glucose. The sources of inflow are the diet
(carbohydrates) and hepatic glycogenolysis. The outflows are to the tissues,
for glycogen synthesis, for energy use, or, if plasma concentrations reach a
sufficient level, into the urine. This
level is not
usually reached in
normal, healthy people.
Regulation of the glucose flows is through the action of
endocrine hormones, these being epinephrine, growth hormone, insulin, glucagon,
glucocorticoids and thyroxine. Insulin is the only hormone with a hypoglycaemic
action, whereas all the others are hyperglycaemic, since they stimulate
glycogenolysis. Thus, falling blood glucose stimulates their release, while
raised glucose stimulates insulin release, an example of dual negative-feedback
control.
Integration of fat, carbohydrate and protein metabolism is
essential for the effective control of the glucose pool. Two other pools are
drawn upon for this, these being the free fatty acid (FFA) pool and the amino
acid (AA) pool (Fig. 44b). The FFA pool comprises the balance between dietary
FFA absorbed from the GIT, FFA released from adipose tissue after lipolysis,
and FFA entering the metabolic process. Insulin drives FFA into storage as
lipids, while glucagon, growth hormone and epinephrine stimulate lipolysis. The
AA pool in the bloodstream comprises the balance between protein synthesis and
the entry of amino acids into the gluconeogenic pathways. A summary of
metabolism is shown in Fig. 44c.
Endocrine control of food intake
The discovery of the hormone leptin,
which is secreted from adipose tissue and which inhibits feeding behaviour in
rodents, has stimulated an interest in the role of the neuroendocrine system in
feeding behaviour and the occurrence of obesity. There is now evidence for a
feedback system in the hypothalamus (see Chapter 45). In humans, food intake is
determined by a number of factors, including the peripheral balance between
usage and storage of energy, and by the brain, which through its appetite and
satiety centres can trigger and terminate feeding behaviour (Fig. 44a). Leptin
is secreted by human adipocytes but it may be more important (in the human) in
the long-term maintenance of adequate energy stores during periods of energy
deficit, rather than as a short-term satiety hormone.
Feeding behaviour in humans can be
initiated and sustained not only through hunger, but also through an awareness
of the availability of especially palatable foods and by emotional states; the
central mechanisms underlying this behaviour are poorly understood. Conversely,
feeding behaviour can be deliberately suppressed, as in anorexia nervosa, when
the patient fasts regardless of the knowledge of the consequence of this
behaviour. There is, however, a growing body of evidence that in some families
there may be genetic contributions, for example mutations of the gene that
expresses the melanocortin-4 receptor (MCR-4) gene has been described in rare
families with obesity in which satiety is not recognized. The relative
contributions of cultural, genetic and non-transmissible factors in the
development of obesity are shown in Fig. 44d.