Chronic Renal Allograft Dysfunction
Chronic, progressive loss of allograft function beginning months or years
after transplant may have a number of causes, both immunological and
non-immunological. Previously, the terms chronic rejection or chronic allograft
nephropathy were used to describe this gradual attrition of graft function.
However, the most recent Banff classification advises distinguishing chronic
antibody-mediated rejection (as evidenced by vascular changes and persistent
C4d staining on biopsy in the presence of donor-specific antibodies [DSA]) from
interstitial fibrosis and tubular atrophy (which can be caused by a number of
factors, including chronic hypertension and CNI).
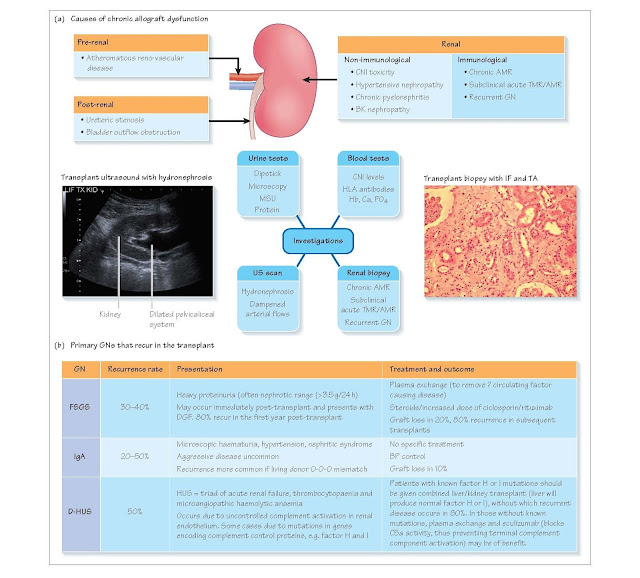
Non-immunological chronic allograft dysfunction
Causes
1 Pre-renal causes:
•
atheromatous vascular
disease
•
hypertension (in donor
and/or recipient).
2 Renal causes:
•
Calcineurin inhibitor
(CNI) toxicity
•
BK virus nephropathy
•
recurrent pyelonephritis
•
diabetic nephropathy.
3 Post-renal:
•
ureteric obstruction
•
bladder outflow obstruction.
Many of these factors are modifiable (e.g. recipient
hypertension, CNI toxicity), therefore it is important to identify them as
early as possible by taking a careful history and performing a detailed
examination.
History and examination
A history of recurrent urinary tract infections (UTIs)
and other urological symptoms should be sought; medications should be reviewed,
with particular attention given to CNI dose, and to nephrotoxins such as
non-steroidal anti-inflammatory drugs (NSAIDs). A history of smoking and
diabetes together with the presence of arterial/transplant bruits, raises the
possibility of atheromatous disease affecting the graft. Current blood pressure
(BP) should be assessed, as well as a review of previous BP. Patients with
chronic urinary obstruction may have a palpable bladder.
Investigations
Blood tests
· Sequential serum creatinine measurement (to estimate rate of decline in
renal function).
· CNI levels (current and historical).
· HLA antibody screen (the presence of DSA would suggest an immunological
cause of graft dysfunction).
Urine tests
· Urine dipstick/analysis – proteinuria/albumin–creatinine ratio (ACR) or
protein-creatinine ratio (PCR).
· Urine cytology – decoy cells in BK nephropathy.
· Mid-steam urine (MSU).
Radiological investigations
· Ultrasound (US): hydronephrosis indicative of obstruction; dampened Doppler
flow suggestive of transplant renal artery stenosis.
· MAG3 – a mercaptoacetyltriglycine radionuclide scan to confirm obstruction
if US suspicious.
· Renal transplant angiogram – if arterial stenosis suspected.
Renal biopsy
If the above investigations do not reveal an obvious
cause for the decline in graft function, then the patient should proceed to a
transplant biopsy to exclude an immunological cause of graft dysfunction such
as chronic antibody-mediated rejection (AMR) and recurrent glomerulonephritis
(GN).
Commonly observed chronic histological changes include
interstitial fibrosis (IF) and tubular atrophy (TA), which are graded according
to the amount of cortical area involved:
In addition to IF/TA, there is frequently vascular
damage, with intimal thickening and glomerulosclerosis. More specific features
of CNI toxicity include tubular cell vacuolation, arteriolar hyali- nosis and
thrombotic microangiopathy.
Management
This depends on the cause. Arterial stenoses should be
treated with angioplasty where possible; ureteric obstruction resolved via
stent insertion and surgical intervention; and bladder outflow obstruction
treated via catheter insertion and/or treatment of prostatic disease. More
general measures include tight blood pressure control (<130/80 mmHg), treatment of proteinuria with ACEi/ ARB, and treatment of chronic
kidney disease-associated anaemia and bone-mineral disease. Where CNI toxicity
is suspected, CNIs may be minimised or even withdrawn, with conversion to
sirolimus (which is non-nephrotoxic).
Immunological chronic allograft dysfunction Causes
1. Chronic AMR
2. Subclinical acute TMR or AMR
3. Recurrent GN
History, examination, and investigation
Recurrent disease
· Review the cause of renal
failure; is it a GN known to recur in transplants (e.g. focal segmental
glomerulosclerosis [FSGS], IgA)?
Rejection
· Have there been episodes
of acute rejection previously, particularly steroid-resistant rejection or AMR?
· Compliance to
immunosuppression should be assessed, both by direct questioning and by
reviewing longitudinal CNI levels.
· The presence of current
or previous DSA increases the likeli- hood of chronic AMR, as does a high
degree of HLA mismatch. Diagnosis ultimately requires a renal transplant
biopsy. Chronic AMR is evidenced by diffuse peritubular capillary (PTC) C4d
staining, transplant glomerulopathy (double contouring in peripheral capillary
loops) and PTC basement membrane multi-layering.
Management
Chronic AMR has no proven treatment. Switching immunosuppression to include tacrolimus and mycophenolate may be helpful. Rituximab is
also being trialled in patients with chronic AMR but the prognosis remains
poor, with 50% loss of graft within 5 years. Subclinical TMR and AMR should be
treated as described in Chapter 23.
Recurrent GNs are seldom amenable to treatment, with the
exception of FSGS or atypical/diarrhoea-negative haemolytic uraemic syndrome
(D-HUS), which can be treated with plasma exchange or eculizumab (atypical
HUS).