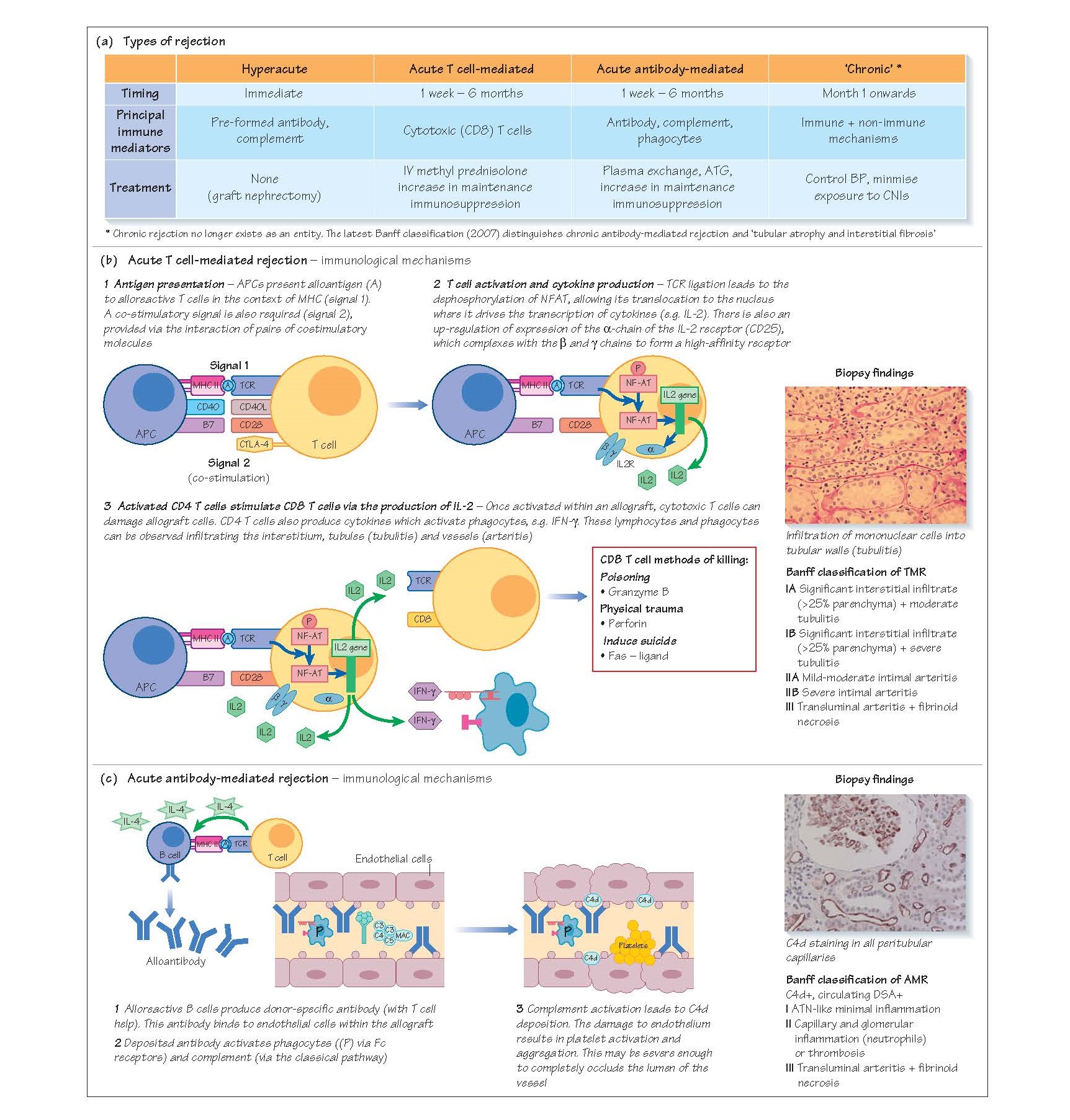
Transplant Rejection
Immunologically mediated allograft damage or rejection
may be hyperacute, acute or chronic. Acute rejection is classified as acute
cellular/T cell-mediated rejection or acute antibody-mediated/ humoral
rejection, according to which arm of the immune system is principally involved
in mediating allograft damage.
Hyperacute rejection
Hyperacute rejection occurs immediately post-transplant
(within minutes to hours) in recipients who have pre-formed, complement-fixing
donor-specific antibodies (DSA, typically ABO or HLA). On perfusion of the
transplant with the recipient’s blood, these antibodies bind to endothelial
cells activating complement and phagocytes. This results in endothelial damage,
platelet aggregation and rapid arterial and venous thrombosis with subsequent
allograft infarction. Once initiated, the process is essentially untreatable,
and inevitably leads to allograft loss. Historically, the first attempts at
transplantation were performed across blood groups, leading to hyperacute
rejection and rapid graft loss. In the current era, hyperacute rejection is
very rare, and usually only occurs if there is a mistake in performing the
cross-match or transcribing a blood group.
Acute cellular rejection
The most common type of rejection is acute cellular
rejection (also known as T cell-mediated rejection [TMR]), occurring in 20–25%
of transplants, usuallywithinthefirst 6 monthspost-transplant. Patients present
with unexplained deterioration in transplant function should undergo an
ultrasound scan to exclude obstruction, a urine dipstick and culture to exclude
infection, and should have their CNI levels assessed to exclude toxicity. If no
alternative cause for decline in graft function is identified, a transplant
biopsy is performed.
Immunological mechanisms
TMR occurs when there is presentation of donor antigen to
recipient CD4 T cells by antigen-presenting cells (APCs), which may be donor-or
recipient-derived (direct antigen presentation = donor MHCI/II/APC; indirect
antigen presentation = recipient MHC Class II/APC; see Chapter 9).
Following antigen presentation, and the provision of co-stimulation through the
interaction of surface pairs of co-stimulatory molecules, activated CD4 T cells
provide help to CD8 (cytotoxic) T cells, phagocytes and B cells, leading to
their infiltration into the graft. Cytotoxic T cells damage
anddestroytargetcells viatheproductionofperforinandgranzyme, and through the
induction of Fas/Fas ligand-mediated apoptosis.
Biopsy findings
Renal allograft pathology is categorised according to the
Banff classification. This is a set of guidelines devised by an international
consortium of transplant histopathologists who originally met in the Canadian
city of Banff. They are regularly updated to incorporate advances in techniques
and in the understanding of pathophysiology.
TMR can affect the tubules and interstitium, causing an
inter-stitial lymphocytic infiltrate and tubulitis (Banff 1 TMR) and, in more
severe cases, an arteritis (Banff 2 TMR).
Treatment
The treatment for TMR is high-dose steroid (e.g. 0.5–1 g
boluses of methyl prednisolone on three successive days). Baseline maintenance
immunosuppression is also increased to prevent recurrent rejection. Most
(80–90%) episodes of acute cellular are amenable to treatment with
corticosteroids. If the patient’s creatinine does not fall in response to
corticosteroids (steroid-resistant TMR) then further treatment with a
lymphocyte-depleting agent such as anti-thymocyte globulin (ATG) is undertaken.
ATG causes profound lymphopaenia, therefore maintenance doses of
anti-proliferative agents (azathioprine or mycophenolate) should be omitted
during the 10–14 days of ATG administration.
Acute antibody-mediated rejection
Acute antibody-mediated rejection (AMR) occurs in around
2–4% of transplants. The diagnosis requires:
·
a decline in allograft
function
·
the presence of
donor-specific HLA antibodies
·
the presence of C4d in
peritubular capillaries (PTC) on biopsy
· the presence of acute
tissue injury (e.g. capillaritis) on biopsy. Recent studies suggest that
non-HLA antibodies, including those recognising major histocompatibility
complex class I-related chain A and B antigens (MICA and MICB) and angiotensin
II type I receptor may also have an adverse impact on allograft outcomes.
Immunological mechanisms
DSA are produced by terminally differentiated B cells,
either short-lived plasmablasts or long-lived bone marrow plasma cells. These
antibodies bind to endothelium and activate complement via the classical
pathway. Deposited antibody will also activate phagocytes with Fc receptors,
including neutrophils.
Biopsy findings
C4d (a degradation product of C4) can be identified on
peritubular capillaries and may be focal (<50% of PTCs) or diffuse (>50% of
PTCs). Peritubulary capillaries may also contain inflammatory cells
(capillaritis) or there may be a more severe arteritis.
There is an increasing, but unresolved, debate about
whether peritubular C4d staining in the absence of graft dysfunction has prognostic
significance and warrants treatment.
Treatment
AMR is treated by removing DSA via plasma exchange or
immu- noadsorption, and preventing antibody-associated inflammation with
corticosteroids and lymphocyte depletion with ATG. The treatment strategy
should also aim to prevent the synthesis of further antibody; however, this is
difficult to achieve with current therapies. In de novo AMR in a
previously non-sensitised patient, some DSA may be produced by short-lived
splenic plasmablasts. These may be reduced by treatment with the CD20 antibody
rituximab, as some of these plasmablasts continue to express CD20, and their B
cell precursors will also be depleted. In sensitised patients, long-lived bone
marrow plasma cells may be the source of antibody, replenished by memory B
cells. These are not amenable to rituximab treatment but DSA-producing plasma
cells may be sensitive to proteosome inhibition with bortezomib.
An alternative to antibody elimination is to block
antibody-mediated graft injury. Eculizumab, an antibody against the C5
complement component, is effective in preventing complement-mediated red cell
lysis in patients with paroxysmal nocturnal haemoglobinuria. Recent data
suggest that eculizumab may also be effective in preventing DSA-mediated
complement activation in the allograft. Even with treatment, AMR may result in
chronic allograft damage and is a much more serious condition than TMR.