Anomalies In Number Of Kidneys
Renal agenesis is defined as the complete absence of one
or both pairs of kidneys and ureters. It represents a failure of the ureteric
bud and metanephric mesenchyme to engage in the process of reciprocal induction
and differentiation required for metanephros formation. As a result, both the
kidney and ureter fail to develop. Renal aplasia, in contrast, occurs when
there is abnormal differentiation of the metanephric mesenchyme and ureteric bud
that leads to involution of the kidney, but with persistence of a rudimentary
collecting system.
Because numerous signaling molecules are involved in
normal metanephros development, the range of genetic defects that may cause
renal agenesis is vast and under active investigation. Recent evidence,
however, suggests a prominent role for abnormalities in the GDNF-RET signaling
cascade. GDNF (glial cell line-derived neurotrophic factor) is expressed in the
masses of metanephric mesenchyme lying near the mesonephric (wolffian) ducts. It
binds to RET, a receptor tyrosine kinase, in the mesonephric ducts and induces
formation of the ureteric buds. Mutations in the genes encoding these proteins
prevent formation of the ureteric buds and have been noted in fetuses with
renal agenesis.
In some cases, male fetuses with renal agenesis still
develop normal mesonephric duct derivatives (i.e., the vas deferens, seminal
glands [vesicles], and epididymis), indicating that the underlying
developmental defect affected only ureteric bud branching or metanephric
induction. Fetuses with broader defects, in contrast, have likely sustained insults
to the intermediate mesoderm, which gives rise to both the nephrogenic cords
and genital ridge (see Plate 2-1).
Bilateral renal agenesis. Bilateral renal agenesis, defined as the complete absence of both
kidneys and ureters, is a rare abnormality that occurs in approximately 1 :
5000 to 1 : 10,000 fetuses. Males are affected at least twice as often as
females. There is evidence that a significant number of affected fetuses have
abnormalities in signaling cascades important to renal development, such as the
GDNF-RET pathway described above. A subset of affected fetuses, however, also
have associated abnormalities that suggest a more wideranging defect in caudal
development, such as sirenomelia (fused lower extremities, imperforate anus,
renal agenesis, and abnormal or absent genitalia).
Bilateral renal agenesis is typically diagnosed using
prenatal ultrasound. Normally the fetal kidneys can be visualized starting at
approximately the twelfth week of gestation. In bilateral renal agenesis,
however, no renal parenchyma can be visualized either in the normal renal fossae or
other ectopic locations, such as the fetal pelvis or thorax. The fetal adrenal
glands are in normal position but may appear less flattened because of the lack
of normal compression from the kidneys. Finally, the fetal bladder appears
empty, and the normal cycles of filling and emptying are not seen.
Severe oligohydramnios is a major consequence of
bilateral renal agenesis and may be one of the more notable sonographic
findings. Before 20 weeks of gestation, diffusion of fluid into the amnion
produces a significant
fraction of the amniotic fluid, which there-fore may appear normal in volume
even despite a lack of fetal renal function. After 20 weeks, however, the fetal
kidneys are responsible for producing over 90% of the amniotic fluid. Severe
oligohydramnios at this stage of development is therefore a very sensitive sign
of bilateral agenesis. It is not, however, particularly specific, and other
possible causes including bilateral renal dysplasia, bilateral renal cystic
disease, urinary outflow tract obstruction, premature rupture of membranes, and
fetal demise must be ruled out.
Severe oligohydramnios, irrespective of the cause, has
numerous adverse effects on the fetus. First, the increase in intrauterine
pressure results in physical compression of the growing fetus, which leads to a
blunted nose; low-set, flattened ears that appear enlarged; micrognathia;
prominent infraorbital folds; a prominent depression between the lower lip and
chin; clubbed limbs; and dislocated hips. In addition, the lack of amniotic
fluid causes abnormal development of the skin, which appears loose and
excessively dry. Finally, the increased pressure on the fetal thorax and
decline in circulating amniotic fluid leads to severe pulmonary hypoplasia. The
cause-and-effect relationship between oligohydramnios and these various
sequelae is known as the Potter sequence.
Bilateral renal agenesis is fatal. Forty percent of
affected fetuses die in utero, and the remainder develop severe respiratory
distress shortly after birth. There-fore, if the diagnosis is established using
prenatal ultra- sound, therapeutic abortion is typically recommended. Because
most cases of bilateral renal agenesis are sporadic, the risk of recurrence in
a subsequent pregnancy is low.
Unilateral renal agenesis. Unilateral renal agenesis, defined as the complete absence of one
kidney and its associated ureter, occurs in approximately 1 : 1000 to 1 : 1200
individuals. Males are affected nearly twice as often as females. The
underlying cause is thought to be abnormal interaction between a ureteric bud
and its associated metanephric mesenchyme; however, it is unclear why some
patients develop unilateral agenesis, whereas in others both sides are
affected.
Like bilateral agenesis, unilateral renal agenesis is
often discovered using prenatal ultrasound, which reveals an empty renal fossa
without evidence of ectopic renal parenchyma, such as a pelvic kidney. Unlike
in bilateral agenesis, however, urine production is normal, and therefore
oligohydramnios does not occur. As a result, affected infants are typically born with a
normal appearance and normal pulmonary function.
Many patients with unilateral renal agenesis have
associated abnormalities in other organ systems. Indeed, the absence of a
kidney may not even be discovered until an associated abnormality is
investigated. Many of these abnormalities occur in structures derived from the
mesonephric or paramesonephric ducts, suggesting a defect in the intermediate
mesoderm early in development. In males, the ipsilateral mesonephric duct derivatives
(vas deferens, seminal gland [vesicle], and epididymis) may be absent or
rudimentary (i.e., a seminal gland [vesicle] cyst). Meanwhile, in females, a
common associated anomaly is a unicornuate uterus, in which the side
ipsilateral to the absent kidney is missing. Associated abnormalities may also
occur in the cardiovascular system (e.g., septal or valvular defects) or
gastrointestinal systems (e.g., imperforate anus).
Nearby
musculoskeletal abnormalities may also be seen. In a smaller subset of
patients, unilateral renal agenesis may be associated with a genetic syndrome
that affects numerous organ systems, such as BOR (branchio-oto-renal) syndrome,
Turner syndrome, Fanconi anemia, Kallmann syndrome, VACTERL (vertebral
anomalies, anal atresia, cardiac defects, tracheoesophageal fistula, renal
defects, limb defects), and others.
With a solitary kidney, renal function typically remains
normal, although for unclear reasons there is an increased risk of
vesicoureteral reflux, ureteropelvic junction obstruction, and ureterovesical
junction obstruction. Later in life, some patients may develop renal
insufficiency and proteinuria, likely secondary to hyperfiltration of the solitary
kidney causing focal segmental glomerulosclerosis (see Plate 4-10). Their survival
rate, however, appears to remain similar to that of normal individuals.
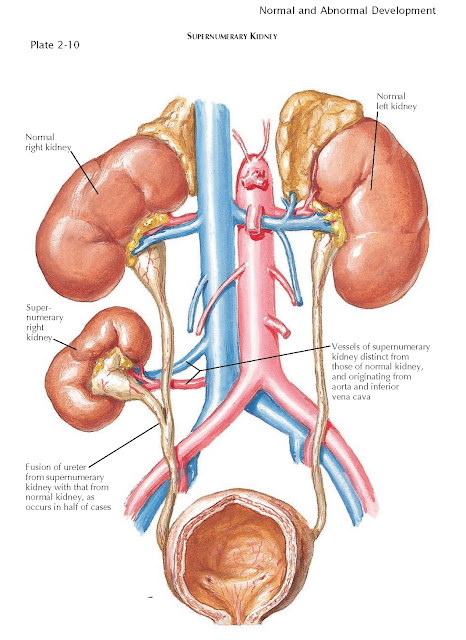
Supernumerary Kidney
A supernumerary kidney is a very rare congenital
anomaly. Unlike a kidney with a duplicated collecting system, which is far more
common, a supernumerary kidney is a distinct mass of renal parenchyma with its
own capsule, vessels, and collecting system. It is typically small and located
just cephalad or caudal to the normally positioned kidney on the same side.
Less commonly, it is located in a variety of other positions, such as the pelvis
or midline. In some cases, the supernumerary kidney and normally positioned
kidney may be loosely attached to each other by either fibrous tissue or a
bridge of renal parenchyma.
In half of cases, the ureter associated with a supernumerary
kidney fuses with that of the normally positioned ipsilateral kidney, as seen
in the illustration; in the other half, the ureter has its own separate
insertion into the bladder. In such cases, the Weigert-Meyer rule is usually
obeyed, meaning that the ureter associated with the more caudally positioned
kidney has an orifice located more superior and lateral than that of the cranially
positioned kidney. The vessels to the supernumerary kidney usually originate
from the aorta and inferior vena cava, although their origin is more variable
with more caudally positioned kidneys.
The embryologic basis of the supernumerary kidney is not
known but likely represents early division of the metanephric mesenchyme. A
supernumerary kidney with a ureter that has its own insertion into the bladder
likely reflects early division of the mesenchyme before insertion and branching
of the ureteric bud (see Plate 2-2). The ureter to the supernumerary kidney
probably represents a second ureteric bud that sprouted from the adjacent
mesonephric duct, either by coincidence or as a direct effect of the divided
mesenchyme. A supernumerary kidney with a ureter that fuses with that of the
normal kidney likely reflects later division of the meta-nephric mesenchyme,
perhaps in response to a ureteric bud that divided before insertion.
Supernumerary kidneys are often asymptomatic and do not
affect overall renal function. Thus a significant number of such kidneys
may never be discovered or may be noted only as incidental findings during the
workup of another unrelated complaint. In some patients, however, supernumerary
kidneys present as palpable abdominal masses or cause symptomatic nephrolithiasis
or an upper urinary tract infection. Because of the rarity of this condition,
affected patients are often not diagnosed until their fourth decade, if at
all.