Male Reproduction Pathophysiology
Clinical background
Male infertility has a large number of
causes, both endocrine and non-endocrine in origin and few are specifically
treatable. In the majority of cases an exact diagnosis is not reached despite
investigation and the condition may result from previous testicular damage,
varicocoele or non-specific inflammation. All patients should be assessed with
their partner in a specialist fertility unit and in the undiagnosed group the
use of intracytoplasmic sperm injection may offer the best chance of fertility.
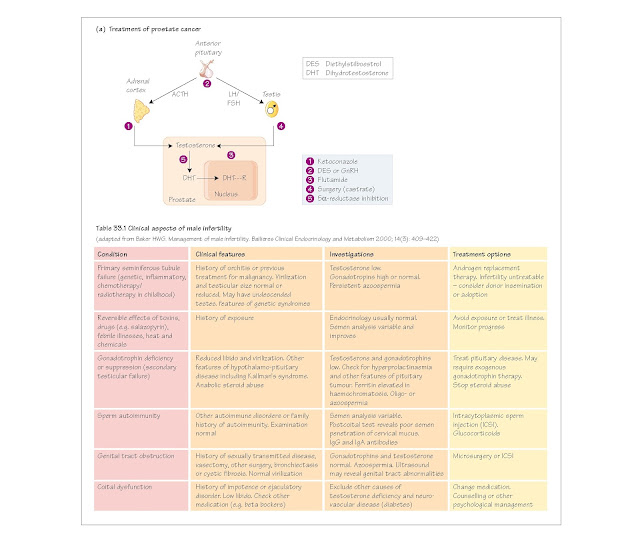
The clinical features of male infertility are shown in Table 33.1.
Male reproductive pathophysiology
Hypogonadism is the failure of
the testes to function, that is to produce testosterone and spermatozoa, and
can be due to genetic defects (see Chapter 23). Primary hypogonadism refers
to abnormalities within the gonad, for example Leydig cell agenesis
(non-development), or failure of Leydig cells in adult life. Leydig cell
failure can occur after mumps. Secondary hypogonadism refers to
gonadotrophin deficiency or failure to secrete gonadotrophin-releasing hormone
(GnRH), and is also called hypogonadotrophic hypogonadism.
Hypergonadism means the excess activity of the gonad, which can
be virilizing due to androgen-secreting Leydig cell tumours, or feminizing
due to estrogen-producing Leydig cell tumours. This is primary
hypergonadism, whereas that pro- duced through excess GnRH and/or
gonadotrophin production is secondary hypergonadism.
Androgen resistance is caused by mutations of the androgen receptor,
which no longer binds androgen with sufficient affinity for a normal androgenic
response to be maintained, or by the complete absence of the androgen receptor.
Gynaecomastia is breast enlargement in males. It usually occurs
through abnormal endogenous or exogenous estrogens. Gynaecomastia accompanied
by galactorrhoea (milk production) may be indicative of a prolactin-secreting
tumour. Gynaecomastia sometimes occurs in ageing men, which may be because of
an increasing estrogen/ androgen ratio in the blood. The condition has also
been reported after the smoking of cannabis, which is known to decrease
testosterone synthesis and to drive down libido.
Impotence (erectile dysfunction) is the failure to achieve
erection of the penis, and has numerous vascular and neurologi- cal causes,
although few of endocrine origin. Erection is caused by nerve impulses passing
through parasympathetic efferents, the nervi erigentes, to the penis. The
result is vasodilatation of penile arteries, which allows the build-up of
arterial blood in the corpus cavernosum and the corpus spongiosum.
The treatment of impotence was
revolutionized by the introduction of sildenafil (Viagra). The drug dilates
penile blood vessels by blocking the enzyme phosphodiesterase-5, which normally
metabolizes the second messenger cyclic GMP, which in turn is permitted to
prolong vascular smooth muscle relaxation in the penis, which is thus engorged
with blood.
Prostatic pathophysiology
Benign prostatic hyperplasia (BPH) is the growth of the medial lobe of the human
prostate, most often in late middleaged men, until it presses on and begins to
occlude the urethra. It is termed ‘benign’ because it does not invade other
tissues and destroy them, or metastasize to distant sites in the body. BPH is
androgen-dependent, being strongly stimulated by dihydrotestosterone (DHT), the
active androgenic metabolite of testosterone in the prostate gland. The most
effective treatment has been the surgical removal of all or part of the gland.
The operation can be performed through the bladder (transvesical prostatectomy)
or through the urethra (transurethral resection), when prostate tissue is
burned away using a heated element. Recently, inhibitors of the enzyme
5α-reductase, which converts testosterone to DHT, have been introduced to treat
BPH.
Prostate cancer. Carcinoma of the prostate is virtually always
androgen-dependent. Various approaches to treatment are shown in Fig. 33a. The
aim is to remove the tumour and all sources of androgen production. Medical
treatment may involve the administration of stable analogues of GnRH, such as buserelin.
These, if continuously present in the bloodstream, down regulate anterior
pituitary production of gonadotrophins by rendering the gonadotrophs
insensitive to GnRH from the hypothalamus. The result is a chemical castration,
which can be reversed by stopping treatment. Another approach is the
administration of androgen receptor blockers such as flutamide, finasteride
or cyproterone acetate.
When using GnRH analogues, it is
advisable when starting treatment to administer the drug together with an
antiandrogen. This is because the initial effect of the GnRH analogue is to
stimulate a transient increase in testosterone production, which may in turn
cause stimulation of tumour activity. Radiotherapy may be necessary as an
adjunctive therapy or for the relief of pain due to metastatic spread.