Female Reproduction Ovarian Steroids
Miss RB, a 24-year-old woman,
presented to her GP complain- ing of increasing hair growth over her chin and
face with greasy skin and acne. The hair growth had been present for 5 or 6
years but over the last 2 years had become much worse, such that she was shaving
her chin three times a week and using a number of depilatory creams purchased
at the chemists. On questioning she revealed that she had also noticed
increased hair growth around her nipples, over her lower abdomen and on the
lower part of her back. Her periods started when she was 12 years old but she
had never had a regular cycle; her periods only occurred every 6–10 weeks and
on one occasion she had missed her period for 4 months. She had always been
‘overweight’ but had gained 2 stones in weight over the last 2 years. Her
mother had had irregular menses and had been treated for subfertility prior to
conceiving RB. Her maternal grandmother had Type 2 diabetes mellitus. On
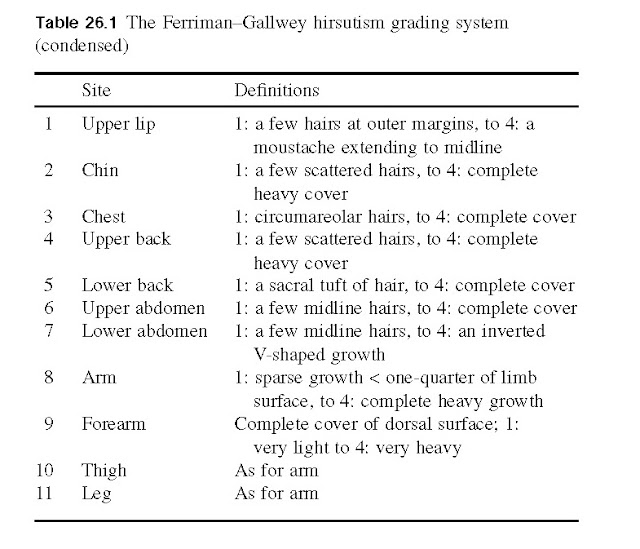
examination RB was found to be obese with a body mass index of 32 kg/m ndrogendependent
hirsutism and a Ferriman and Galwey score was found to be 16. The diagnosis of
polycystic ovary syndrome was confirmed when subsequent biochemical
investigations showed her to have a raised testosterone concentration of 3.2
nmol/L, LH 14.5 U/L, FSH 3.3 U/L and an ultrasound scan of the ovaries showed
bilaterally enlarged ovaries with numerous peripherally sited follicles. After
discussion she was treated with diet, exercise and the drug metformin, all of
which lower insulin resistance and reduce ovarian androgen secretion. This
combination resulted in improvement in the hirsutism and establishment of a
more regular menstrual cycle.
Polycystic ovary syndrome is the
commonest cause of hirsutism and irregular menstrual cycles. Patients have a
long history, usually dating back to the menarche. It is important in the
assessment of women with hirsutism to exclude those with a short history and
features of virilization that might suggest androgen-secreting tumours of the
ovary or adrenal glands.
Physiological actions of estrogens
The effects of the estrogens can be
classified in chronological order of the major reproductive events of the
female (Fig. 26a). Overall, their main influence is on the maintenance of
fertility. Sexual differentiation. During fetal development, estrogens
are not required for the normal differentiation and development of the female
genitalia and accessory sex organs, but they are needed for sexual
differentiation of the brain.
Puberty. During puberty (see Chapter 24), estrogens stimulate development of the breast stroma, endometrium, myometrium and vagina.
Estrogens cause epiphyseal closure and characteristic fat deposition in
peripheral tissues.
Adult. In the adult female, estrogens maintain the
menstrual cycle and female secondary sexual characteristics. Estrogens
facilitate the actions of progesterone by stimulating the synthesis of
progesterone receptors, notably in the brain and uterus.
Pregnancy. During pregnancy, estrogens increase the blood
flow to and through the uterus, they cause hypertrophy of the uterine
myometrium and stimulate breast ductal proliferation. They enhance fluid
retention and stimulate uterine progesterone receptor synthesis. Shortly before
parturition (birth), estrogens stimulate the synthesis of oxytocin receptors in
the uterus myo- metrium. Oxytocin is involved in parturition through its contractile action on the uterus (see Chapter 34).
Metabolic effects. Estrogen inhibits bone resorption, an action which
becomes apparent after the menopause, when estrogen wanes. Estrogens decrease
bowel motility. They affect liver function by stimulating protein synthesis,
including that of sex hormone-binding globulin (SHBG) and thyroxine-binding
globulin (see 13). Estrogens affect blood coagulability by stimulating the
production of factors II, VII, IX, and X but decreasing platelet aggregation.
They have important effects on plasma lipids, decreasing total cholesterol,
increasing high-density lipoprotein (HDL) and decreasing low-density lipoprotein (LDL) concentrations.
Menopause marks the cessation of natural female reproductive
life. The ovaries no longer produce ova, and the secretion of estrogens
declines and eventually ceases. The symptoms asso- ciated with the menopause
vary from individual to individual and between cultural groups. Vasomotor
instability causing hot flushes and sweating, vaginal dryness and an increased
rate of bone resorption, potentially leading to osteopaenia and osteoporosis,
are the only established features of estrogen deficiency and are relieved by
estrogen replacement therapy.
Mechanism of action of estrogens Estrogens travel in the bloodstream, largely bound
to plasma proteins, and diffuse into the cell and the nucleus where they bind
to specific receptor proteins (Chapter 4).
Two main forms of the estrogen
receptor have been discovered, namely ER-α and ER-β. ER-α is the form that
dictates much of sexual function and behaviour, and may be the form of the
receptor responsible for breast and other estrogen-mediated cancers. There is
evidence that the ratio of ER-α: ER-β is an important determinant of health and
disease, especially with regard to carcinogenicity of estrogen. The estrogen
receptor proteins have been characterized and have different multifunctional
domains (Fig. 26b). The receptor has at least two transcriptional activation
functional sites (TAF-1 and 2; see also Chapter 4), a DNA-binding domain, which
is similar for many of the DNA- binding receptors, and a hormone-specific
binding domain.
Ovarian androgens
The ovary is also an important source
of androgen production in the female, accounting for about 50% (the rest being
adrenal in origin). Androstenedione and testosterone are synthesized in the
theca cell layer of the maturing follicle under the influence of LH. They
diffuse into granulosa cells where they are aromatized to form estrogens (Fig.
26c). Androgens and the other steroid and peptide hormones produced in the
developing follicle are important local regulators of ovarian function and
folliculogenesis. In mature females, many of the common disorders of
reproductive function are associated with excessive androgen production,
disordered folliculogenesis and ovulation and subsequent subfertility
associated with the peripheral effects of excess androgen production (Figs 26d
and e).