Cell Signalling Genes
The stem
cells of the embryo are intinially aranged in a very simple ball of cells: a
structure from which they create complex tissues of multiple cell types and
shapes, connected by the systems of the body. The interplay of cells, through
signals produced and received by one another, underpins these processes. One
signal or set of signals can cause a number of effects downstream as the cells
differentiate or begin new interactions with neighbouring cells (see Chapter
3). In this way a surprisingly small number of signalling factors are able to
coordinate the early stages of embryonic development.
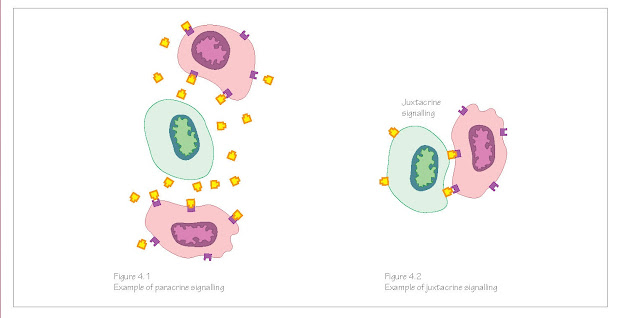
Some signals
can diffuse short distances across embryonic tissues and bind to receptors on
the surfaces of target cells (paracrine signalling, Figure 4.1), and some
signals require direct cell‐to‐cell contact (juxtacrine signalling, Figure
4.2). Transmembrane receptor proteins undergo conformational changes when the
extracellular domain binds a ligand; this modification usually causes the
intracellular portion to dissociate and trigger a cascade of events resulting
in the binding of transcription factors to DNA and changes to gene expression.
Paracrine
signalling is relatively conserved between species and, in general, uses four
groups of signalling proteins, namely Wnt, Hedgehog, fibroblast growth factor
(FGF) and transforming growth factor beta (TGF‐β), all of which are intricately
involved in development. Juxtacrine signalling can occur between adjacent cells
or between a cell and the extracellular matrix. The direct contact and bonds
that form have been shown to be vital in a number of developmental processes,
including the neural system and the heart.
Notch is an
example of a cell membrane protein receptor that once bound to its juxtacrine
ligand on an adjacent cell (another cell membrane protein such as Serrate or
Delta) undergoes a conformational change, is cleaved by an enzyme and the
dissociated intracellular portion binds to a dormant transcription factor that
affects gene expression. A number of varieties of Notch and its associated
ligands are involved in human embryonic development.
Transcription
factors are proteins that can bind to DNA and affect the transcription of
nearby regions. They can promote or inhibit (up‐or downregulate) the
transcription of a particular gene from DNA to mRNA. The Hox proteins are
transcription factors involved in body pattern formation and segmentation and
their genes have been highly conserved during evolution, having similar roles
in very different species (see Chapter 21). To classify as a transcription
factor a protein must contain a section that can bind directly to DNA. There
are other proteins involved in regulating DNA expression that are not
transcription factors as they cannot bind to DNA.
There are
over 200 types of modification that can occur to a protein post‐translation,
but all are involved in determining the biological properties of a protein.
Commonly these include chemical alterations including hydroxylation,
methylation, sulphation, phosphorylation andglycosylation. Altering aprotein
post‐translationally can affect protein shape, activity, turnover, interactions
with other proteins and localization. Through interactions with enzymes these
proteins can have functional groups added or taken away, such as sugars, lipids
and proteins. The post‐translational modification of certain proteins in
signalling pathways ha n to affect cell‐to‐cell interactions during
development.
Wnt protein
are vertebrate version of the Wingless gene that was first identified in
the fruit fly (Drosophila melanogaster), so named because mutating the
gene caused flies to develop without wings. The Wnt signals bind to Frizzled
transmembrane cell surface receptors, and they are involved in body patterning
(see Chapter 21), cell fate, proliferation and migration. Failures of these
systems in humans result in limb, eye, genitourinary and bone development
disorders. Soluble forms of Frizzled known as sFrps (secreted Frizzled‐like
proteins) bind Wnt proteins and inhibit Wnt signalling.
Early
research into Notch was also performed in Drosophila, where this gene was
initially discovered. In development this gene has great importance in cell
differentiation and inducing specific cell clusters that lead to neurone,
endothelial, cardiocyte and T cell development, to name a few. Notch has also
been shown to help maintain stem cell populations.
Hedgehogs
are a family of proteins that work much like a skeleton key (one key that can
open a lot of different doors). Their effect on a cell depends on cell type,
dosage and how differentiated the cell is. The Hedgehog gene was first
identified in Drosophila and in addition to its body patterning role in the
early embryo mutations of this gene caused naturally occurring spiky denticles
to occur in a solid region rather than in stripes, and made the embryo shorter,
giving the embryo a hedgehog‐like appearance. The Hedgehog family in humans
includes Sonic hedgehog homologue (SHH), Indian hedgehog homologue (IHH) and
Desert hedgehog homologue (DHH). Sonic hedgehog has a key role in neural, bone,
limb and kidney development; muscle patterning; and lung branching. It is also
involved in the development of the special sense organs. It binds to a cell
surface transmembrane receptor called Patched.
There are
currently 22 varieties of FGF with a number of func- tions in adult tissues.
During embryological development they are key players in a wide range of
processes including limb and neural development, angiogenesis, very early
patterning and induction of mesoderm development. FGFs bind FGF receptors
(FGFRs) and heparan sulphate proteoglycans are part of the signal transduction
process. The many different FGF types and receptor combinations allow for a
variety of effects in different situations, and interruption of these effects
during development causes a number of developmental abnormalities.
TGF‐β is
described as part of a superfamily of signalling factors, and includes the bone
morphogenetic proteins (BMPs). When originally discovered it was associated
with tumour development, but a number of structurally similar molecules have
since been identified and implicated in many events during embryological
development and in adult tissues. Three forms of TGF‐β and 15 types of BMPs
have been discovered. TGF‐β ligands bind to a type II TGF‐β receptor that
recruits a type I receptor, triggering a SMAD cascade and ultimately a change
in DNA transcription.