Venous
Thromboembolism And Pulmonary Embolism
Venous thromboembolism and its most significan complication, pulmonary
embolism (PE), are common clinical disorders that have a substantial impact
on patient morbidity and mortality; Fig. 28c shows major risks. PE is most
often a complication of deep venous thrombosis (DVT). Both disorders are
commonly underdiagnosed and require appropriate clinical suspicion and a
systematic diagnostic approach. About 5 million patients develop DVT in the USA
each year; approximately 500 000 subsequently develop PE and approximately 10%
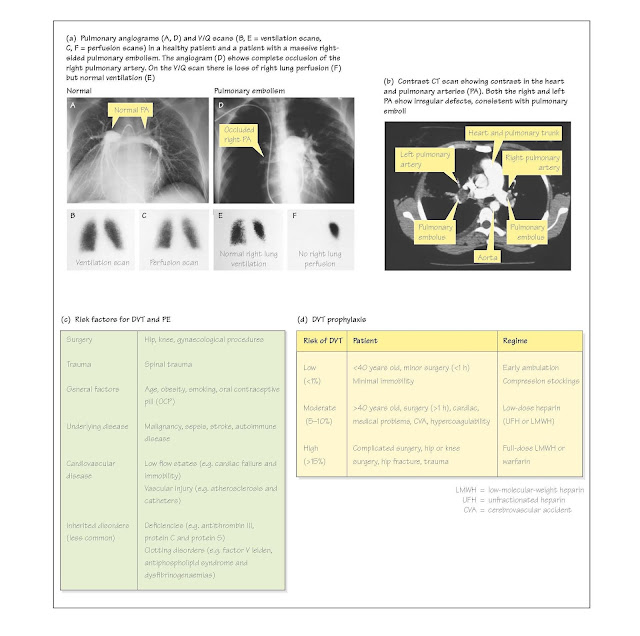
of these die. Prophylactic therapy in patients at risk is essential (Fig. 28d);
in its absence up to 70% of patients undergoing hip or knee replacement surgery
develop DVT.
Deep venous thrombosis
Nearly all clinically significan cases
of PE ( ̴90%) arise from DVT in the lower extremities, with
thrombi typically originating in the calves and propagating above the knee.
Approximately 15-25% will propagate into the femoral and iliac veins and have
a 50% risk of embolizing to the lung. Thrombi may develop in the axillary and
subclavian veins, usually due to surgery or intravenous catheters, but emboli
are usually smaller, with less risk of catastrophic consequences. Soon after
thrombus formation, the intrinsic fibrinolyti cascade begins to organize the
thrombus. The risk of a thrombus embolizing is greatest early during ongoing
proliferation and decreases once it is organized.
Pulmonary embolism
When a thrombus embolizes to the lung,
respiratory or circulatory abnormalities occur due to sudden occlusion of a
pulmonary artery or arteriole. Occlusion of regional perfusion causes an
increase in dead space, necessitating an increase in minute ventilation to
maintain normal Paco2. Surfactant production
distal to the embolus may be reduced after 24 hours, resulting in atelectasis.
Hypoxaemia is common and mostly due to VA/Q
mismatch (Chapter 14). Pulmonary infarction occurs in less than 25%
of cases of PE. Circulatory complications arise from obliteration of the
pulmonary vascular bed and a reduction of cardiac output. Severity is related
to the amount of lung embolized and the pre-existing state of the pulmonary
vasculature and right ventricle (RV). A single large embolus can be
catastrophic, whereas multiple small emboli can cause 'pruning' of smaller
arteries. Circulatory collapse may occur with more than 50% obstruction of the
pulmonary vascular bed. Less severe emboli may be fatal to patients with pre-
existing lung or heart disease.
Clinical features
Clinical features of DVT are
non-specific with lower extremity pain, swelling and erythema. Homan's sign
(pain in the calf on dorsifl xion of the foot) occurs in a minority of
patients. Fifty per cent of DVTs are undetected.
Most patients with PE have dyspnoea,
pleuritic chest pain, haemoptysis, apprehension and tachypnoea.
With severe PE, signs related to RV failure (e.g. hypotension and
jugular venous distension) may occur. Most patients with PE have non-specifi
abnormalities on chest X-ray, including atelectasis. The electrocardiogram
(ECG) may show non-specifi ST segment changes, and rarely, with significan RV strain,
an S1Q3T3 pattern (prominent S in lead I, Q
and inverted T in lead III), right axis deviation (RAD) or right bundle-branch
block (RBBB). Arterial blood gas abnormalities are common, including widened
A–a gradient, hypoxaemia and hypocapnia (despite increased
dead space).
Diagnosis
Deep venography or pulmonary angiography is the diagnostic
standard, although V/Q scanning is usually the initial investigation as
it is less invasive (Fig. 28a; Chapter 21). A negative perfusion scan
effectively rules out PE and a 'high probability' scan (multiple segmental
perfusion defects with normal ventilation) has a more than 85% probability of
PE (Fig. 28a). With a high clinical suspicion, a high-probability V/Q scan has
a positive predictive value of more than 95%. Unfortunately, most V/Q scans are
non-diagnostic or indeterminate, with a 15-50% likelihood of PE, necessitating
further imaging. Non-invasive imaging of the lower extremity deep veins
with Doppler imaging or impedance plethysmography is useful, because the
presence of thrombosis requires treatment similar to PE. In patients with
underlying cardiac or pulmonary disease, pulmonary angiography is
indicated if the above tests are non-diagnostic. Absence of DVT and a low
probability V/Q scan permit treatment to be withheld. Spiral/helical
computed tomography (CT) has a sensitivity for PE of 70-95% (higher for
more proximal emboli) and a specificit of more than 90%. It also allows
visualization of parenchymal abnormalities and is used in patients with chronic
obstructive pulmonary disease (COPD) or extensive chest X-ray abnormalities,
where V/Q scanning is indeterminate. Echocardiography may reveal RV
dysfunction in PE and rule out pericardial tamponade or severe left ventricular
(LV) dysfunction. Transoesophageal echocardiography may visualize
thromboemboli in the main pulmonary arteries, but not in lobar or segmental
arteries.
Treatment
The cornerstone of therapy for DVT/PE
is anticoagulation, which stops propagation of existing thrombus and
allows organization. Immediate therapy in patients with a high suspicion of PE
may prevent further life-threatening embolization. Standard therapy is to give unfractionated
heparin (UFH) or low-molecular-weight heparin (LMWH) for 5-7 days,
followed by warfarin for 3-6 months. UFH and warfarin must be monitored
as subtherapeutic levels increase the risk of recurrent thromboembolism. LMWH
is more bioavailable and does not require monitoring. Patients with inherited
or acquired hypercoagulability may require lifelong therapy.
In patients with contraindications to
anticoagulation (recent surgery, haemorrhagic stroke, central nervous system
metastases, active bleeding) or recurrent PE while on therapeutic
anticoagulation, an inferior vena cava (IVC) filter may prevent fatal PE.
Although activation of fibrinolysis with
thrombolytics hastens resolution of perfusion defects and RV
dysfunction, convincing benefi is lacking. As thrombolytics cause increased
bleeding complications, including a 0.3-1.5% risk of intracerebral haemorrhage,
they are only recommended for life-threatening PE with compromised
haemodynamics.