The Special Cases Of
Syphilis And Human Immunodeficiency Virus
Natural history of untreated
syphilis
Syphilis is
caused by the spirochete bacterium, Treponema pallidum, which
enters the body through miniscule breaks in the skin of the external genitalia
that occur during sexual intercourse. Once the spirochete has entered, the
untreated disease progresses through four consecutive stages: primary,
secondary, latent and tertiary syphilis. Antibiotic treatment at any stage
short of tertiary can prevent the late, life-threatening sequelae of the
disease. Syphilis may also be transmitted from a woman to her fetus at any
point during pregnancy, with serious consequences.
The primary
lesion of syphilis, the chancre, develops in venereal locations close to
where T. pallidum typically enters the body: the penis, labia, perineum,
anus or rectum. Chancres are painless, small papules that persist for 1–2
months and heal spontaneously.
The
secondary stage of syphilis is a disseminated form. Blood- borne spirochetes
populate the dermis throughout the body causing a widespread papular rash over
the trunk and extremities. Because the disease is systemic, fever, myalgias,
lymphadenopathy, sore throat and headache are common. Secondary syphilis can
also be associated with immune complex deposition in the joints, kidneys and
eyes, leading to arthritis, glomerulonephritis, nephrotic syndrome and uveitis.
Untreated secondary syphilis resolves over 4–12 weeks, leaving the patient symptom
free. The subsequent months to years until the onset of symptoms of tertiary
syphilis is known as the latent period.
Tertiary
syphilis usually appears many years after the disseminated stage. Tertiary
syphilis can involve multiple organs, including the cardiovascular and nervous
systems. Overall, about one-quarter of untreated patients develop recognizable
late (tertiary) complications of syphilis, one-quarter have asymptomatic
lesions demonstrable at autopsy and half have no anatomic lesions attributable
to syphilis present at autopsy.About half of the patients with symptomatic
tertiary syphilis will die as a direct result of the disease, typically of
cardiovascular complications.
Infection of
the placenta and fetus will occur in virtually 100% of pregnant women who
suffer the spirochetemia accompanying primary or secondary syphilis.
Complications of syphilis in pregnancy include miscarriage, stillbirth,
premature delivery and congenital syphilis. The manifestations of congenital
syphilis are protean. Its neonatal mortal- ity rate is 50%. Syphilis is treated
with penicillin in all but highly allergic patients.
Epidemiology of syphilis
Syphilis was
very common in many parts of the world until antibiotic therapy became
available in the 1940s. The prevalence of the disease fell dramatically after
World War II but began to increase again in the 1960s. Up to 75% of cases go
unreported. Women and men at high risk for contracting syphilis are young, from
lower socioeconomic groups, and have multiple sexual partners. Some 10–50
syphilitic organisms are sufficient to cause infection and about one-third of
the sexual contacts of an infected person will become infected. The incidence
of congenital syphilis parallels that in women and is increasing. Mandatory prenatal
screening has reduced the incidence of late congenital syphilis; late or absent
prenatal care is the biggest risk factor for congenital syphilis.
Biology of T. pallidum
Treponema
pallidum is a member of the bacterial order Spirochaetaceae, and closely
related to two other treponemas responsible for human disease: Treponema
pertenue, which causes yaws, and T. cara- teum, which causes pinta.
Neither electron microscopic examination nor DNA analyses can distinguish
between these three organisms. It is believed that the different diseases that
develop reflect adaptations of the organism and the host to different points of
entry into the body. Treponema pallidum is a relatively fragile organism
that cannot survive for more than a few hours outside moist areas of the body.
Its microbiology is very poorly understood because the organism cannot be
maintained in cell culture.
Most of the manifestations of
syphilis are secondary to the inflammatory reaction caused by the organism. Polymorphonuclear
cells arriving at the site of the inoculum ingest the spirochetes but do not
kill them. Lymphocytes and macrophages are recruited to the site. They also
surround, but do not kill the treponemes. Antitreponemal antibodies are
produced, sometimes in quantities that cause immune complex glomerulonephritis.
It remains both amazing and unknown how T. pallidum is able to evade
host defenses and establish an infection. The site of primary infection is
surrounded by a mucoid material composed of hyaluronic acid and chondroitin
sulfate that may alter the host defenses. The best clue available to explain
the persistence of disease is the finding that delayed type sensitivity to
treponemal antigens is absent in secondary syphilis. New spirochetes inoculated
into the system are not infectious while the original infection persists. This
is a common mechanism in chronic parasitic diseases, called “premunition”; the
host resists reinfection but cannot clear the initial infection.
Once the
systemic phase of the infection is established, spirochetes are present
virtually everywhere in the infected tissues. However, inflammation occurs
preferentially around small vessels and causes intimal hyperplasia and
obliterative endarteritis. The subsequent focal ischemic necrosis and fibrosis
are responsible for the many late manifestations of the disease.
The
inflammatory changes caused by the spirochetes are most striking in congenital
syphilis. The placenta is diffusely
fibrotic with inflammation and necrosis of the fetal blood vessels in the
placental villi. The resulting vascular insufficiency leads to poor fetal
growth (intrauterine growth restriction) and stillbirth. Fibrosis
of the liver and spleen cause fetal anemia. Compensatory extramedullary hematopoeisis promotes hepatosplenomegaly and the development of pleural effusions
and ascites (fetal hydrops). Some infants will have a skin rash that
closely resembles that of secondary syphilis. A runny nasal discharge loaded
with spirochetes (snuffles) may be the only hint of congenital syphilis
at birth.
The late
manifestations of syphilis, both congenital and tertiary, involve vasculitis
and parenchymal damage in the central nervous system.
Human immunodeficiency virus
Natural history of untreated HIV
infections
The first
description of human disease associated with HIV infection surfaced in the
early 1980s. Acute infection was reported to cause a “mononucleosis-like
syndrome” with fever, malaise, muscle aches, headache, fatigue, generalized
rash, sore throat, lymphadenopathy and characteristic mucocutaneous lesions.
The rapidity of symptom onset after initial contact
may reflect the route of viral entry and the viral load of the exposure.
Symptoms of primary infection often persist for 2–3 weeks before resolving
spontaneously. The disease then enters an asymptomatic phase. This can last
from several months to many years. The length of this symptom-free phase
appears to depend on the pathogenicity of the infecting viral strain.
Coinfection with other viruses or other sexually transmitted disease (STD)
pathogens may speed disease progression. During the asymptomatic phase, viral
replication continues within infected lymphoid cells (mainly CD4+ T cells).
Infected immune cells are destroyed by the virus and, eventually, the host
becomes immunocompromised. In this immunocompromised state, the HIV-infected
individual is vulnerable to a variety of opportunistic viral, bacterial, fungal
and parasitic infections. Oppor-tunistic pathogens such as Pneumocystis
carinii, Cryptosporidium and Cryptococcus seldom affect
individuals with normally functioning immune systems but can be deadly in those
infected with HIV. Patients who are severely immunocompromised are also at risk
for the development of certain neoplasms, including Kaposi sarcoma, human
papillomavirus-related cervical cancers and some lymphomas. The development of
opportunistic infections or neoplasms in a patient infected with HIV defines
the acute immunodeficiency syndrome (AIDS). Patient who die of AIDS typically
succumb to complications of an opportunistic infection or neoplasm.
Epidemiology of HIV infections
HIV has
infected over 60 million people worldwide, and 35 million are presently living
with the disease. The developing world accounts for 95% of infections, with
over 25 million of those presently infected living in sub-Saharan Africa. The
most important risk factor for acquiring HIV infection and succumbing to its
complications is poverty.
Viral transmission
occurs through direct contact with bodily fluids, most often semen or blood.
Viral spread can occur via sexual contact, via parenteral exposure (intravenous
drug abuse and transfusions) or via
perinatal transmission. The latter
can occur during
pregnancy (transmission across the placenta), at delivery or during
breastfeeding. Only 25% of children born to untreated HIV-positive mothers will
acquire the infection, although this rate can be decreased to less than 1–2%
with aggressive antenatal and perinatal therapy. Over 90% of HIV
infections occur via
heterosexual transmission. HIV is
more readily transmitted from the male to female (1 in 500–1000 acts of receptive
vaginal intercourse) than female to male (1 in 2000–2500 acts of insertive
vaginal intercourse).
Biology of HIV
HIV is a
retrovirus. Its genetic material is carried as RNA wrapped in a viral protein
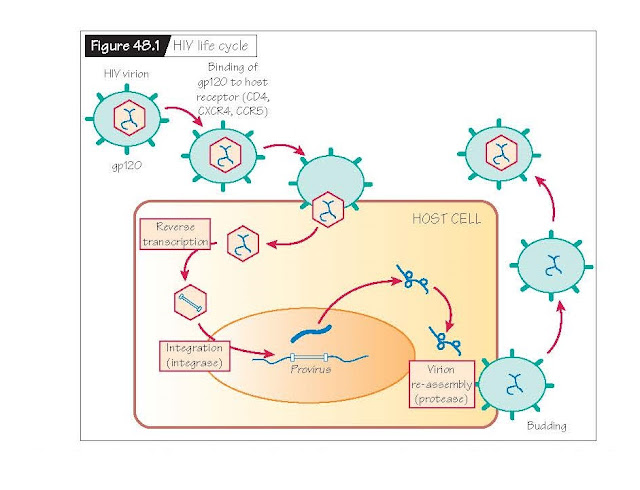
coating. The viral surface expresses a receptor called gp120 that binds
specifically to receptors on lymphoid cells (Fig. 48.1). Binding promotes viral
entry into host cells. Host receptors and co-receptors for viral entry include
CCR5, a chemokine receptor on macrophages, CXCR4, a chemokine receptor
expressed on T cells, and CD4, a marker for T helper cells that is also
expressed on macrophages and dendritic cells. Once viral entry has occurred,
infected cells will fuse with CD4+ T helper cells. Viral propagation will
continue largely in CD4+ cells.
After entry
into a host cell, the retrovirus uses reverse transcriptase to make a DNA copy
of its viral RNA genome. The virus then uses an enzyme called integrase to
insert its newly synthesized DNA into the host genome and the host cell
machinery makes multiple copies of the HIV genome. The virus finally employs an
enzyme called protease to reassemble the viral envelope. Viral particles then
exit the host cell via budding to infect surrounding receptor-laden immune
cells. Multiple viral progeny will be produced within a single infected host
cell before it expires.
Reverse
transcriptase (RT), integrase and protease are virus-specific enzymes. They can
therefore serve as targets for directed therapeutic interventions. Over 20
FDA-approved medications are now available to treat HIV infections. None are
curative and optimal therapies typically use combinations of two to four medications.
Available antiretroviral medications inhibit each of the HIV-specific enzymes:
the HIV protease (protease inhibitors), the RT enzyme [nucleoside RT inhibitors
(NRTI), non-nucleoside RT inhibitors (NNRTI)], and HIV integrase (integrase
inhibitors). Inhibitors of HIV viral entry have recently been released.
In developed
countries, careful therapeutic interventions, combined with close monitoring of
CD4+ T-cell counts and viral loads, have radicallyimprovedtheprognosisforthoseinfectedwith
HIV. Furtheradvances are challenged by the fact that the HIV reverse
transcriptase enzyme makes many mistakes during replication of the viral
genome. The virus has no way to readily correct these mistakes. This allows for
rapid viral mutation and, unfortunately, the development of resistance to
antiretroviral medications. In underdeveloped countries, where the prevalence
of disease is highest, medications are scarce or completely unavailable.