Nutritional And
Metabolic Aspects Of Iron
The transport and storage of iron
is largely mediated by three proteins: transferrin, transferrin receptor 1
(TfR1) and ferritin.
Transferrin molecules can each contain
up to two atoms of iron. Transferrin delivers iron to tissues that have
transferrin receptors, especially erythroblasts in the bone marrow which
incorporate the iron into haemoglobin (Figs 2.7, 3.2). The transferrin is then
reutilized. At the end of their life, red cells are broken down in the
macrophages of the reticuloendothelial system and the iron is released from
haemoglobin, enters the plasma and provides most of the iron on transferrin.
Only a small proportion of plasma transferrin iron comes from dietary iron,
absorbed through the duodenum and jejunum.
Some iron is stored in the macrophages
as ferritin and haemosiderin, the amount varying widely according to overall
body iron status. Ferritin is a water‐soluble protein–iron complex. It is made
up of an outer protein shell, apoferritin, consisting of 22 subunits and an
iron–phosphate–hydroxide core. It contains up to 20% of its weight as iron and
is not visible by light microscopy.
Haemosiderin is an insoluble
protein–iron complex of varying composition containing approximately 37% iron
by weight. It is derived from partial lysosomal digestion of ferritin molecules
and is visible in macrophages and other cells by light microscopy after
staining by Perls’ (Prussian blue) reaction (see Fig. 3.10). Iron in ferritin
and haemosiderin is in the ferric form. It is mobilized after reduction to the
ferrous form. A copper‐containing enzyme, caeruloplasmin, catalyses oxidation
of the iron to the ferric form for binding to plasma transferrin. Iron is also
present in muscle as myoglobin and in most cells of the body in iron‐containing
enzymes (e.g. cytochromes or catalase) (Table 3.1). This tissue iron is less
likely to become depleted than haemosiderin, ferritin and haemoglobin in states
of iron deficiency, but some reduction of these haem‐containing enzymes may
occur.
Regulation of ferritin and
transferrin
receptor 1 synthesis
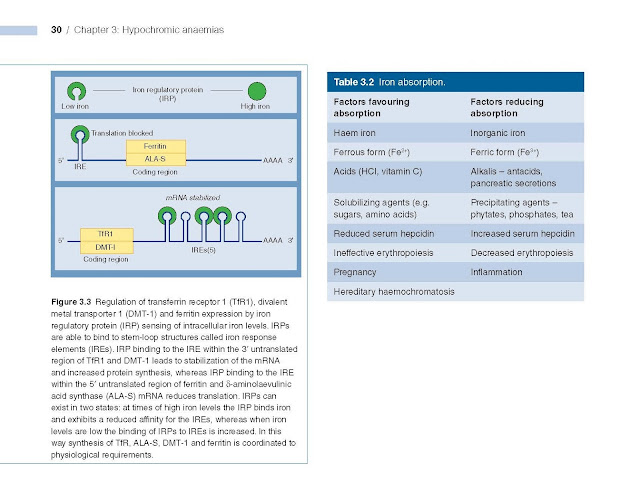
The levels of ferritin, TfR1, δ‐aminolaevulinic
acid synthase (ALA‐S) and divalent metal transporter 1 (DMT‐1) are linked to
iron status so that iron overload causes a rise in tissue ferritin and a fall
in TfR1 and DMT‐1, whereas in iron deficiency ferritin and ALA‐S are low and
TfR1 increased. This linkage arises through the binding of an iron regulatory
protein (IRP) to iron response elements (IREs) on the ferritin, TfR1, ALA‐S and
DMT‐1 mRNA molecules. Iron deficiency increases the ability of IRP to bind to
the IREs whereas iron overload reduces the binding. The site of IRP binding to
IREs, whether upstream (5′) or downstream (3′) from the coding gene, determines
whether the amount of mRNA and so protein produced is increased or decreased
(Fig. 3.3). Upstream binding reduces translation, whereas downstream binding
stabilizes the mRNA, increasing translation and so protein synthesis.
When plasma iron is raised and
transferrin is saturated, the amount of iron transferred to parenchymal cells
(e.g. those of the liver, endocrine organs and heart) is increased and this is
the basis of the pathological changes associated with iron loading conditions.
There may also be free iron in plasma which is toxic to different organs (see
Chapter 4).
Hepcidin
Hepcidin is a polypeptide produced by
liver cells. It is the major hormonal regulator of iron homeostasis (Fig.
3.4a). It inhibits iron release from macrophages and from intestinal epithelial cells by its
interaction with the transmembrane iron exporter, ferroportin. It accelerates
degradation of ferropor tin mRNA. Raised hepcidin levels therefore profoundly
affect iron metabolism by reducing its absorption and release from macrophages.
Control Of Hepcidin Expression
Membrane‐bound hemojuvelin (HJV) is a
co‐receptor with bone morphogenetic protein (BMP) which stimulates hepci din
expression (Fig. 3.4b). A complex between HFE and trans ferrin receptor 2
(TfR2) promotes HJV binding to BMP. The amount of HFE–TfR2 complex is
determined by the degree of iron saturation of transferrin as follows. Diferric
transferrin competes with TfR1 for binding to HFE. The more diferric
transferrin, the less TfR1 is bound to HFE and more HFE is available to bind to
TfR2, with consequently increased hepcidin synthesis. Low concentrations of
diferric transferrin, as in iron deficiency, allow HFE binding to TfR1,
reducing the amount of HFE able to bind TfR2 and thus reducing hepcidin
secretion. HFE also increases BMP expression, directly increas ing hepcidin
synthesis.
Matriptase 2 digests membrane‐bound
HJV. In iron deficiency, increased
matriptase activity
therefore results in decreased hepcidin synthesis.
Erythroblasts secrete two proteins, erythroferrone and GDF 15, which suppress
hepcidin secretion. In conditions with increased numbers of early erythroblasts
in the marrow (e.g. conditions of ineffective erythropoiesis, such as
thalassaemia major), iron absorption is increased because of suppression of
hepcidin secretion by these proteins. Hypoxia also suppresses hepcidin
synthesis, whereas in inflammation interleukin 6 (IL‐6) and other cytokines
increase hepcidin synthesis (Fig. 3.4a).
Dietary iron
Iron is present in food as ferric
hydroxides, ferric–protein and haem–protein complexes. Both the iron content
and the proportion of iron absorbed differ from food to food; in general meat,
in particular liver, is a better source than vegetables, eggs or dairy foods.
The average Western diet contains 10–15 mg iron daily from which only 5–10% is
normally absorbed. The proportion can be increased to 20–30% in iron deficiency
or pregnancy (Table 3.2) but even in these situations most dietary iron remains
unabsorbed.
Iron absorption
Organic dietary iron is partly
absorbed as haem and partly broken down in the gut to inorganic iron.
Absorption occurs through the duodenum. Haem is absorbed through a receptor,
yet to be identified, on the apical membrane of the duodenal enterocyte. Haem
is then digested to release iron. Inorganic iron absorption is favoured by
factors such as acid and reducing agents that keep iron in the gut lumen in the
Fe2+ rather than the Fe3+ state (Table 3.2). The protein
DMT‐1 is involved in transfer of iron from the lumen of the gut across the
enterocyte
microvilli
(Fig. 3.5). Ferroportin at the basolateral surface controls exit of iron from
the cell into portal plasma. The amount of iron absorbed is regulated according
to the body’s needs by changing the levels of DMT‐1 and ferroportin. For DMT‐1
this occurs by the IRP/IRE binding mechanism (Fig. 3.3), and for ferrroportin
by hepcidin (Fig. 3.4a).
Ferrireductase present at the apical
surface converts iron from the Fe3+ to Fe2+ state and
another enzyme, hephaestin (ferrioxidase), converts Fe2+ to Fe3+
at the basal surface prior to binding to transferrin.
Iron requirements
The amount of iron required each day
to compensate for losses from the body and for growth varies with age and sex;
it is highest in pregnancy, adolescent and menstruating females (Table 3.3). Therefore these groups are
particularly likely to develop iron deficiency if there is additional iron loss
or prolonged reduced intake.