Endocrine autoimmunity
A 25-year-old woman, Mrs WG, was
referred to the local endocrine clinic. She had visited her GP complaining of
increasing tiredness and fatigue and, over the 4 weeks prior to presentation,
had noticed she felt giddy at times, particularly when she got out of bed in
the morning or on standing up from a chair. Mrs G was known to have primary
hypothyroidism, on thyrox-ine replacement therapy and vitiligo over her
forearms and chest. Her most recent set of thyroid function tests were in the
normal range. The GP had noted her blood pressure to be 90/45. At the clinic
the hypotension was confirmed and on questioning she had noticed increased
pigmentation over her knees and around her waistband. A short Synacthen test
was performed during which her basal plasma cortisol level was found to be 75
nmol/L and 100 nmol/L 30 minutes after injection of 250 μg of synthetic ACTH
(Synacthen). Later her basal ACTH concentration was reported at 550 ng/L and
adrenal antibodies were positive, confirming the diagnosis of primary adrenal
failure. She was started on glucocorticoid replacement in the form of
hydrocortisone and mineralocorticoid replacement with fludrocortisone,
following which her symptoms rapidly improved.
Many endocrine conditions have an
autoimmune aetiology and patients frequently exhibit antibodies to multiple
endocrine organs and have evidence of associated autoimmune disease such as
pernicious anaemia, depigmentation of the skin (vitiligo; Fig. 22a) or
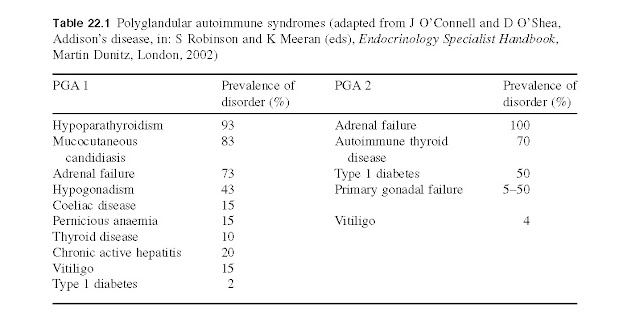
coeliac disease. Two specific autoimmune polyglandular syndromes are recognized
in which there are two or more affected endocrine glands as well as
non-endocrine manifestations (Table 22.1):
·
PGA 1 presents
in children and is an autoimmune recessive disorder;
· PGA 2 (also known
as Schmidt’s syndrome) is a familial disorder most commonly seen in women and thought
to be HLA DR3 linked.
Autoimmunity
Autoimmunity may be defined as an
attack by the host’s immune system on the host’s own tissues. These attacks may
be transient immune reactions to infection, for example, which resolve
spontaneously. They may, however, become chronic, with pathological consequences.
Endocrine autoimmunity often involves an immune attack on specific endocrine
glands, for
example Addison’s disease, Graves’
disease, Hashimoto’s thyroiditis and insulin-dependent diabetes mellitus,
where the gland is damaged or destroyed altogether. These are examples of
mainly organ-specific autoimmune diseases (Fig. 22b). In systemic autoimmune
disease, on the other hand, the immune system attacks several tissues that may
be anatomically distant from each other. Examples of systemic autoimmune
disease include rheumatoid arthritis, scleroderma and systemic lupus
erythematosus (SLE). There may be both organ-specific and systemic components
in most, if not all, autoimmune diseases. Some autoimmune diseases may have
genetic and/or endocrine components, since some, notably Graves’ disease
(thyrotoxicosis), Hashimoto’s thyroiditis, rheumatoid arthritis (RA) and SLE,
are more prevalent in women, and the sex hormones, especially estrogens, may be
important mediating factors.
Mechanisms of autoimmunity. These are not well understood at the moment, but
three important mechanisms have been defined so far: (i) direct
antibody-mediated; (ii) T cell- mediated; and (iii) immune complex-mediated
(Fig. 22c). While autoimmune diseases might tentatively be classified in terms
of these three mechanisms, it is possible that all three are involved in an
autoimmune disease.
1. Direct antibody-mediated disease: Graves’ disease is an example of direct antibody
action on a gland causing damage. The disease can be passively transferred from
a diseased to a healthy organism by the transfer of IgG antibodies. For
example, babies born of mother swho have untreated Graves’diseas eexhibit symptoms
of thyroiditis until the baby’s system destroys the IgG which had been
transferred via the placenta. In severe cases, the baby may be successfully
treated using plasma exchange.
2. T cell-mediated disease: Hashimoto’s thyroiditis is an example of this
type of endocrine autoimmunity (Fig. 22d). In these patients, autoreactive T
cells cause tissue damage in the thyroid by two main mechanisms: (i) they
recruit and activate macrophages, which destroy tissues; and (ii) T cells release
cytokines, for example tissue necrosis factor (TNF). Possibly, suppressor T-cell
function is impaired in these patients, and helper T cells inappropriately
stimulate autoantibody production in B cell, including the production of TSH
receptor antibod- ies, which bind to the TSH receptor on thyrocytes. In addition
to these T cell-mediated effects, iodine uptake and thyroglobulin binding may
be directly interfered with by autoreactive antibodies. Furthermore, the
inflammation caused by autoimmune reactions may trigger apoptosis in
thyrocytes. Thyrocytes, unusually, constitutively express the FAS receptor
ligand, which combines with the FAS receptor to cause apoptosis of the
thyrocytes.
3. Immune complex-mediated disease: systemic autoimmune diseases, such as SLE, are
most probably caused by immune complex-mediated reactions. Patients with SLE
have several circulating autoantibodies to both cytoplasmic and nuclear constituents,
for example IgG directed against double-stranded nuclear DNA. The cytoplasmic and
nuclear antigens may not themselves be pathogenic; a major pathogenic event is
the deposition of the immune complexes in tissues such as the kidneys.
Genetic factors. Epidemiological and familial studies of virtually
all autoimmune diseases point to a genetic susceptibility. The most important
genetic determinant appears to be the major histocompatibility complex (MHC), a
series of genes on chromosome 6 that code for antigens, including the human
leukocyte antigen (HLA) system. Recent research suggests that there are
multiple genetic loci that contribute to autoimmune diseases such as
insulin-dependent diabetes mellitus (IDDM). In the case of IDDM, the gene
encoding preproinsulin may be a locus for genetic polymorphism that may be
associated with susceptibility to IDDM.
Endocrine factors. The possible role of endocrine hormones, for
example estrogens, in the aetiology of autoimmune disease is unknown at
present, but the sexual dimorphism of the distribution of several autoimmune
diseases points to the involvement of the sex hormones. This putative role for
sex hormones is given support from the well-known phenomenon of RA remission
during pregnancy, and the rebound exacerbation or ‘flare’ of disease after
parturition. SLE, as mentioned above, is far more common in women, especially
during the reproductive years, and often flares up during pregnancy and after
parturition. SLE may be precipitated or flare after commencement of oral
contraceptive use. It has been reported that patients with SLE and their
first-degree relatives had elevated serum levels of 16α-hydroxyestrone, which
is an actively feminizing metabolite of estradiol.