Diffuse Parenchymal
(Interstitial) Lung Diseases
Diffuse parenchymal (interstitial)
lung disease (DPLD/ILD) describes a group of disorders characterized by
inflammatio and/or fibrosi of the pulmonary interstitium (i.e. tissue between
the alveolar epithelium and capillary endothelium) and the bronchovascular and
septal tissues, comprising the lung's f brous framework. Alveolar airspaces,
distal airways and vasculature may also be involved.
Clinical features: Insidious onset of dyspnoea and cough, bilateral
inspiratory crackles ( ± clubbing) and exercise-induced desaturation are
common to all DPLD. Hypoxaemia and right-sided heart failure occur in advanced
disease. Pulmonary function tests (PFT) reveal a restrictive defect with
reduced total lung capacity (TLC), functional residual capacity (FRC) and
residual volume (RV) due to impaired lung compliance. Gas transfer (DLco)
is decreased due to diminished surface area for gas exchange. Chest X-ray (CXR)
is abnormal (>90%) with mainly lower lobe alveolar, interstitial or
mixed infiltrates Bronchoalveolar lavage (BAL) excludes other diseases
(e.g. malignancy). An increase in BAL inflammator cells indicates alveolitis
and corre- lates with ground glass opacificatio (GGO) on high resolution computed tomography (HRCT) scans and may reflec rapidly progressive or potentially
reversible disease. HRCT scans ( ± histology) are required classification
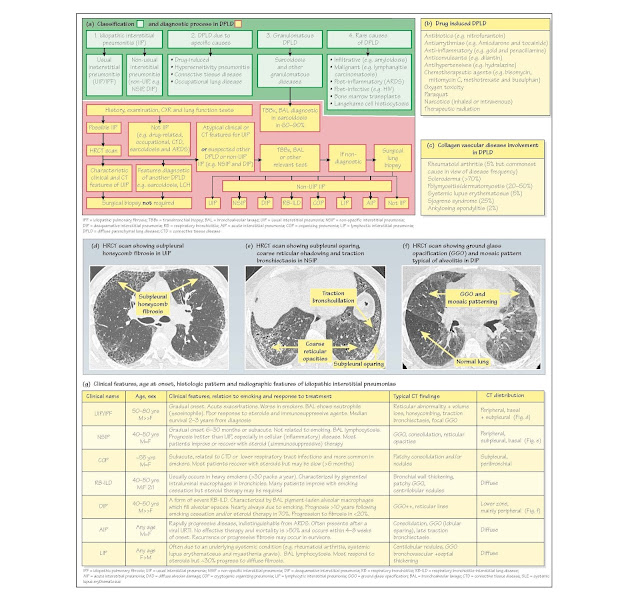
Classification: There are four main categories of DPLD (Fig. 30a)
with considerable overlap:
1 Idiopathic interstitial
pneumonitis (IIP) has two
subgroups:
· Usual interstitial pneumonia (UIP; previously known as idiopathic pulmonary
fibrosi (IPF) or cryptogenic f brosing alveolitis), causes approximately 70% of
IIP. Pathogenesis involves minimal inflammatio with fibrosi due
to fibroblas proliferation and abnormal alveolar epithelial healing. Incidence
is approximately 5/105/year. It usually
occurs in older men, aged
approximately 70 years. Clinical features include progressive dyspnoea,
cough, clubbing (25-50%) and basal inspiratory crepitations interspersed with
acute 'exacerbations'. HRCT scans show bilateral, basal and
subpleural reticular changes with honeycombing and/or traction bronchiectasis
(Fig. 30d). Consolidation, GGO and nodules are infrequent. Histology reveals
peripheral, patchy damage, f brosis and honeycombing alternating with areas of
normal lung. A similar picture occurs in asbestosis, collagen vascular and
drug-induced diseases. Diffuse alveolar damage (DAD) and cryptogenic organizing
pneumonia (COP) occur during acute exacerbations. Treatment: UIP
does not respond to steroids or immunosuppressants. Median survival from
diagnosis is <3 years and worse in smokers.
· Non-usual interstitial pneumonitis (non-UIP) causes approximately 30% of IIP.
Differences in clinical course, histology, HRCT and outcome suggest that these
rare disorders are distinct clinico- pathologic entities. They include (in
order of frequency) non-specifi interstitial pneumonitis (NSIP), COP, previously
known as bronchiolitis obliterans organising pneumonia (BOOP), acute
interstitial pneumonitis (AIP; formerly known as Hamman-Rich syndrome),
respiratory bronchiolitis-interstitial lung disease (RB-ILD), desquamative
interstitial pneumonitis (DIP) and lymphoid interstitial pneumonitis (LIP).
Clinical and radiological features, treatment response and prognosis are
summarized in Fig. 30g. Presentation is earlier, mainly in men, aged 40-50
years. Pulmonary involvement is diffuse, (with subpleural sparing), less f
brotic and more cellular (Fig. 30e) than UIP. HRCT scans may show bilateral
GGO, which may be widespread, subpleural or basal (Fig. 30f). About 50% of
cases are sensitive to steroid ( immunosuppressive) therapy with a median
survival of >10 years. However, prognosis may be poor in
steroid-resistant disease (e.g. AIP ≤6 months).
2 DPLD due to specific causes include
· Drug-induced DPLD (Fig. 30b). Mechanisms include oxidantmediated
injury (e.g. nitrofurantoin), direct cytotoxic effects (e.g. bleomycin),
cellular phospholipid deposition (e.g. amiodarone) and immune-mediated injury
(e.g. hydralazine). Treatment includes drug withdrawal and occasionally steroid
therapy. Irreversible damage may cause respiratory failure (e.g. amiodarone).
· Hypersensitivity pneumonitis (HP; also known as extrinsic al- lergic
alveolitis) is discussed in Chapter 33. It is an inflammator response to
inhaled, mainly organic antigens, to which the patient has become sensitized
(e.g. thermophilic actinomycetes in mouldy hay).
· Connective tissue disease (CTD) DPLD occurs in 5% of rheumatoid arthritis (RA)
patients, especially those with multisystem disease (e.g. vasculitis and
nodules) but is usually asymptomatic. Symptomatic DPLD occurs in many other CTD
(Fig. 30c) and after some treatments (e.g. methotrexate and gold). NSIP is the
predominant histological pattern except in RA, where 50% have a UIP pattern.
· Occupational lung disease follows inhalation of mainly mineral (e.g. coal
and asbestos) dusts (Chapter 33).
3 Granulomatous DPLD: sarcoidosis, the second most frequent DPLD, and
other granulomatous DPLD are discussed in Chapter 31. 4 Rare causes of DPLD include
infiltrat ve (e.g. amyloidosis), ma- lignant (e.g. lymphangitis
carcinomatosis), post-inflammator (e.g. ARDS and vasculitis), post-infective
(e.g. HIV), bone marrow transplantation and rare lung diseases (e.g.
Langerhan's cell histiocytosis).
Diagnosis (Fig. 30a): DPLD due to occupational exposure, drugs, CTD, HP
or sarcoidosis is often diagnosed after a comprehensive his- tory (i.e.
occupational exposure), careful examination (e.g. for CTD), blood tests (e.g.
rheumatoid factor), serology (e.g. avian precipitans), PFT and radiological
imaging. In contrast, initial diagnosis of IIP is one of exclusion. Subsequent
classification to distinguish UIP from other IIP (e.g. NSIP), has important
therapeutic and prognostic implications and requires an integrated clinical,
radiological and pathological approach. HRCT scans aid
differentiation. Typical clinical and HRCT features allow confiden diagnosis of
UIP (sensitivity 43-78%, specificit 90-97%) and avoid the need for biopsy in
50% of cases. Surgical biopsy is considered if radiology is not
diagnostic and in non-UIP cases. Transbronchial biopsies are usually inadequate
for histological classificatio but may be diagnostic in sarcoidosis.
Treatment is
considered in patients with rapidly deteriorating symptoms, inflammator changes
(e.g. GGO) or if requested despite poor evidence for benefi (e.g. UTP).
Supportive therapy requires supplemental oxygen, pulmonary rehabilitation,
nutrition, smoking cessation and palliative care. Pharmacological therapy
includes steroids and/or immunosuppressive agents (e.g. azothioprine,
methotrexate and cyclophosphamide). Tn steroid-sensitive conditions (e.g. COP,
DTP and NSTP), a short trial of high-dose steroids may be indicated. However,
in patients requiring ongoing therapy or less likely to respond (e.g. UTP),
combination 'triple' therapy with low-dose prednisolone, azothioprine and N-acetylcysteine
(NAC) is usually recommended. Tn many patients, therapy is ineffective and has
significan side effects. Consider early referral for lung transplantation.