Acute Coronary Syndromes: ST Segment Elevation Myocardial
Infarction
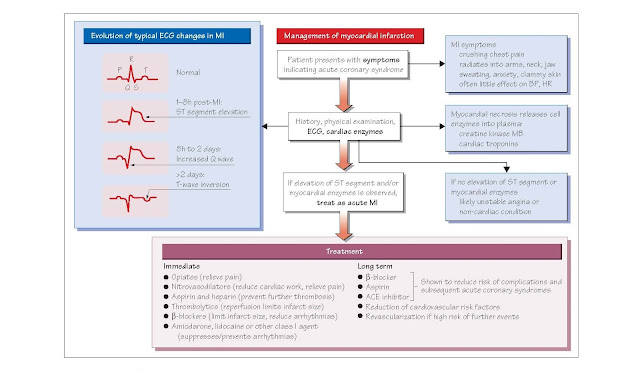
Patients usually present with sudden onset central
crushing chest pain, which may radiate down either arm (but more commonly the
left) to the jaw, back or neck. The pain lasts longer than 20 min and is not
relieved by glyceryl trinitrate (GTN). The pain is often associated with
dyspnoea, nausea, sweatiness and palpitations. Intense feelings of impending
doom (angor animi) are common. Some individuals present atypically, with
no symptoms (silent infarct, most common in diabetic patients with
diabetic neuropathy), unusual locations of the pain, syncope or pulmonary
oedema. The pulse may demonstrate a tachycardia or bradycardia. The blood
pressure is usually normal. The rest of the cardiovascular system examination
may be unremarkable, but there may be a third or fourth heart sound audible on
auscultation as well as a new and/or worsening murmur, which may be due to
papillary muscle rupture in the left heart.
· ECG: ECG changes associated with myocardial infarction (MI) indicate the
site and thickness of the infarct. The first ECG change is peaking of the T
wave. ST segment elevation then follows rapidly in a ST elevation myocardial
infarction (STEMI).
·
Troponin I: elevated plasma concentrations of troponin I indicates that myocardial
necrosis has occurred. Troponins begin to rise within 3–12 h of the
onset of chest pain and peak at 24–48 h and then clear in about 2 weeks. It is
important that a troponin level is interpreted in the clinical context, because
conditions other than MI can damage cardiac muscle (e.g. heart failure,
myocarditis, pericarditis, pulmonary embolism or renal failure). Patients
presenting with suspected acute coronary syndromes (ACS) should have troponin
measured at presentation. If it is negative, it should be repeated 12 hours
later. If the 12 h troponin is also negative, then MI but not unstable angina
can be excluded.
Management
Immediate In the
ambulance or on first medical contact, individuals with suspected MI are
immediately given 300 mg chewable aspirin and 300 mg clopidogrel to
block further platelet aggregation. Two puffs of GTN are sprayed
underneath the tongue. The patient is assessed by brief history and a clinical
examination, and a 12-lead ECG is recorded. The patient is given oxygen via a
face mask. Morphine, which has vasodilator properties, together with an
anti-emetic (e.g. metoclopramide) is administered to relieve pain and anxiety,
thus reducing the tachycardia that these cause. A β-blocker (e.g.
metoprolol) should be given unless contraindicated (e.g. LV failure or moderate
to severe asthma) because β- blockers decrease infarct size and have a positive
effect on mortality. The preferred treatment of a confirmed STEMI is revas cularization
with percutaneous coronary intervention (see Chapter 43) of the
blocked artery within 2 h of symptom onset. Ideally, every hospital would be
equipped with the ability to perform per- cutaneous coronary intervention (PCI)
but in reality this is not the case. However, those that do not have the
capacity for PCI are affiliated with centres that do and protocols exist to
enable the rapid transfer of patients. If it is not possible to get the patient
to a centre for PCI in less than 2 h from symptom onset, the alternative is
pharmacological dissolution of the clot with thrombolytic agents within 12 h of
presentation unless contraindicated (see below). There are specific ECG
criteria for the diagnosis of STEMI and use of thrombolysis: ST segment
elevation of >1 mm in two or more limb leads or >2 mm in two or more
chest leads, or new onset left bundle branch block, or posterior
changes (ST depression and tall R waves in leads V1–V3). If thrombolysis
fails, the patient must be sent for rescue PCI to be performed as soon as
possible.
Subsequent Long-term
treatment with aspirin, a β-blocker and an angiotensin-converting enzyme
inhibitor (ACEI) reduces the com- plications of MI and the risk of
reinfarction. Cessation of smoking, control of hypertension and diabetes, and
reduction of lipids using a statin (see Chapter 36) are vital.
Thrombolysis is the
dissolution of the blood clot plugging the infarct-related coronary artery. As
described in Chapter 43, thrombolytic agents induce fibrinolysis, the
fragmentation of the fibrin strands holding the clot together. This permits reperfusion
of the ischaemic zone. Reperfusion limits infarct size and reduces the risk
of complications such as infarct expansion, arrhythmias and cardiac failure.
Clinical trials, notably ISIS-2 (1988), have demonstrated that thrombolytic
agents reduce mortality by about 25% in STEMI, although patients without ST
elevation (i.e. NSTEMI) do not benefit from thrombolysis. It is critical
that thrombolysis is instituted as quickly as possible. Although significant
reductions in mortality occur when thrombolytics are given within 12 h of
symptom onset, the greatest benefits occur when therapy is instituted within 2
h (‘time is muscle’).
The two main agents for thrombolysis are streptokinase
(SK) and tissue plasminogen activator (tPA) (see Chapter 43). tPA
appears to have a slight survival benefit over SK, but the former is much less
expensive. tPA is very quickly cleared from the plasma, and reteplase and
tenecteplase are newer agents that have been made by modifying the
structure of tPA in order to impede plasma clearance. Both can therefore be
given by bolus injection by para- medics, thus facilitating prehospital
thrombolysis.
The main risk of thrombolysis is bleeding, particularly
intracerebral haemorrhage, which occurs in ∼1% of cases. Contraindications to thrombolysis therefore include
recent haemorrhagic stroke, recent surgery or trauma, and severe hypertension.
Other drugs used in acute myocardial infarction Antiplatelet and anticoagulant therapy is used after MI to prevent further
platelet aggregation and thrombosis. The ISIS-2 trial demonstrated a 23%
reduction in 35-day mortality in patients randomized to aspirin. Combined
aspirin and SK had an additive benefit compared with placebo (42% reduction).
Following an initial 300 mg loading dose, 75 mg/day aspirin should be given
thereafter for life to all patients to prevent vessel occlusion and infarction.
Because tPA, reteplase and tenecteplase are more fibrinspecific than SK,
intravenous heparin should be given for a duration of 48–72 h to reduce the
risk of further thrombosis when these agents are administered, or when the patient
is at high risk of developing systemic emboli (e.g. with anterior MI or atrial
fibrillation). β-Blockers are beneficial in MI for several reasons. They
diminish O2 demand by lowering heart rate and decrease ventricular
wall stress by lowering afterload. They therefore reduce ischaemia and infarct
size when given acutely. They also decrease recurrent ischaemia and free wall
rupture, and suppress arrhythmias (see Chapter 48). Long-term oral β-blockade reduces
mortality, recurrent MI and sudden death by about 25%.
ACEI (e.g. lisinopril,
ramipril) reduce afterload and ventricular wall stress and improve ejection
fraction. Inhibition of ACE raises bradykinin levels, which may improve
endothelial function and limit coronary vasospasm. ACEI also limit ventricular
remodelling and infarct expansion (see Chapter 47), thereby reducing mortality
and the incidence of congestive heart failure and recurrent MI. Therapy should
be instituted within 24 h in patients with STEMI, especially if there is
evidence of heart failure or left ventricular dysfunction, and should continue
long term if LV dysfunction remains evident.
Complications of acute myocardial infarction
There are two main groups of complications associated
with acute MI: mechanical and arrhythmic.
With large infarcts (>20–25% of the left ventricle)
depression of pump function is sufficient to cause cardiac failure. An
infarct involving more than 40% of the LV causes cardiogenic shock. Rupture of
the free LV wall is almost always fatal. Severe LV failure (cardiogenic shock)
as a result of MI is heralded by a large fall in cardiac output, pulmonary
congestion and often hypotension. The mortality is extremely high. Treatment
involves O2 to prevent hypoxaemia, diamorphine for pain and anxiety,
fluid resuscitation to optimize filling pressure and positive inotropes
(e.g. the β1-agonist dobutamine) are infused to aid myocardial
contractility. Revascularization is crucial. Intraortic balloon counterpulsation
can be used temporarily to support the circulation. A catheter-mounted
balloon is inserted via the femoral artery and positioned in the descending
thoracic aorta. The balloon is inflated during diastole, increasing the
pressure in the aortic arch and thereby improving perfusion of the coronary and
cerebral arteries. During systole, deflation of the balloon creates a suction
effect that reduces ventricular afterload and promotes systemic perfusion.
Rupture of the ventricular septum creates a ventricular
septal defect (VSD) and may result in leakage of blood between the ventricles.
Rupture of the myocardium underlying a papillary muscle, or more rarely of the
papillary muscle itself, may cause mitral regurgitation, detected
clinically as a pansystolic murmur radiating to the axilla. Dressler’s
syndrome is the triad of pericarditis, pericardial effusion and fever.
Arrhythmias in the acute
phase include ventricular ectopic beats, and the potentially life-threatening
broad complex (QRS complex >0.12 s) tachyarrhythmias, ventricular
tachycardia (VT) or ventricular fibrillation (VF). Supraventricular arrhythmias
include atrial ectopics, atrial flutter and atrial fibrillation. Bradyarrhythmias
are also common, including sinus bradycardia, and first-, second and thirddegree
AV block. Infarct expansion (see Chapter 44) is a dangerous late
complication.