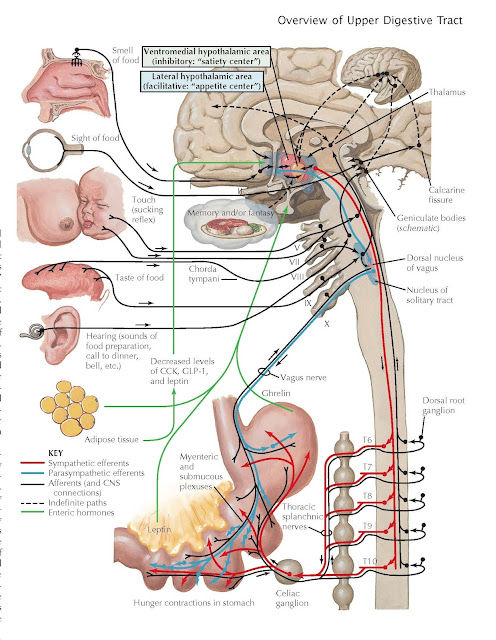
Hunger and Appetite
Food intake is due to
a complex interplay of emotional factors, learned behaviors, CNS regulation,
fat cell thermoregulatory effects, and the digestive system. It has become
increasingly clear that the intake of food is influenced by what appears to be
an adipose “set point,” which results in a relatively stable weight in most
individuals despite efforts to change their weight. The ingestion of food
occurs in response to need (hunger) or desire (appetite). Hunger describes
the complex behavioral responses evoked by depletion of body nutrient stores
required for metabolic needs. Studies by Pavlov and his colleagues in the early
1900s emphasized the importance of cortical functions and the vagus nerve
through learned behaviors and their associations with food intake. The fact
that food-seeking behavior is manifested in the unconditioned state, as in
newborn or anencephalic infants or decerebrate animals, emphasizes the
important role of lower brain functions, including the reticuloactivating
system and hypothalamus.
The digestive system has a major influence on appetite
and hunger. A common sensation described by patients as hunger is discomfort
localized to the epigastrium and perceived as emptiness, gnawing, or tension.
The fact that such ‘hunger pangs’ are experienced by individuals whose stomach
has been removed or denervated is evidence that hunger contractions are
not simply related to gastric contractions. On the other hand, it is clear that
the stomach is the major source of the hormone ghrelin, which is an
important stimulant of food intake. This 28 amino acid peptide is released from
X/A-like cells in the oxyntic glands of the gastric fundus but is also found in
the pancreas and small intestine. It is structurally related to motilin. Its
release leads to increased gastric smooth muscle contractions and stimulation
of CNS appetite centers that stimulate food intake.
Anorexia is not a common
symptom in patients with complete denervation of the small intestine, as occurs
in small bowel transplantation. On the other hand, hormones released as part of
the phenomenon known as the ileal brake have a significant influence on
appetite, including peptide YY3-36, which suppresses food intake.
Surprisingly, basal levels and postprandial levels of peptide YY are decreased
in obese patients.
A variety of other gut neuropeptides influence food
intake. Neuropeptide Y, released from the pancreas as well as the hypothalamus,
increases food intake. Insulin can also increase food intake. Cholecystokinin,
released primarily from the duodenum, reduces food intake.
In addition to influences from the CNS and digestive
system, adipose tissue also regulates appetite. The key appetite suppressant leptin
is synthesized and released from adipose tissues. Leptin is a hormone with
extraordinarily broad influences on metabolism, growth, angiogenesis, and other functions;
it appears to primarily serve as the satiety hormone. Leptin modifies
appetite primarily through its release by white adipose tissue but is also
synthesized and released by brown adipose tissue, skeletal muscles, the placenta,
ovaries, mammary epithelial cells, and bone marrow. In the digestive tract, it
is released by cells in the gastric fundus and by gastric chief cells. It acts
as an internal modulator of energy homeostasis, metabolism, and cell
replication. Although its primary effects are thought to be mediated by its
effects on the hypothalamus, especially on serotonin cells, there are leptin
receptors throughout the body on many types of cells. It is clear that leptin
release is suppressed by fasting, well before fat stores per se are altered,
and is increased by stress, insulin, and corticosteroids and, paradoxically, in
obese persons.
Fat stores, particularly of brown adipose tissue, also
influence appetite. Brown adipose tissue plays a major role in thermogenesis
and appears to be regulated by the CNS hormone orexin. It may be more
involved with energy expenditure than appetite per se. Orexin is a neuropeptide
hormone structurally related to the gut hormone secretin. It is also released
from the hypothalamus and is responsible for both arousal and appetite. It
increases lipogenesis. In addition to the hypothalamus, it is also present in
neurons throughout the CNS. Orexin release is inhibited by leptin and increased
by action of the gastric hormone ghrelin. Decreased orexin can lead to a
feeling of lowered energy which may cause a person to eat more to acquire
energy. Such reflexive food intake in the setting of reduced energy expenditure
can contribute to obesity.